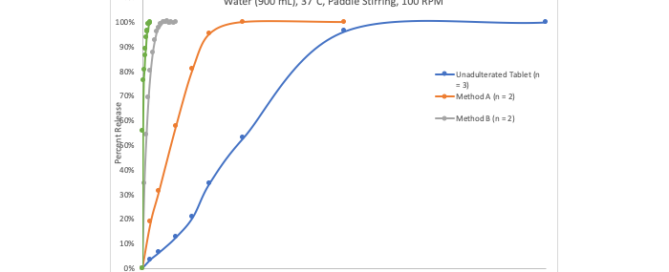
Recent research on bedaquiline selected for the November cover of Acta Crystallographic
Improved Pharma is pleased to announce the open-source publication of research into finding new salts of bedaquiline, an important drug for the treatment of tuberculosis. The drug is currently marketed in the US as the fumarate salt. Our team discovered several new salts, and the crystal structures of two benzoate salts are described in Read more