Salt screens are commonly used to alter the physicochemical properties of a compound for development into an acceptable drug product. Some properties that can be affected include dissolution rate, solubility, hygroscopicity, stability and even toxicity. As is typical for many pharmaceuticals, the solubility of a compound may be too low, ultimately resulting in poor bioavailability. In other instances, a salt may be desired to improve developability of the parent compound which could be of great value to process chemists and formulators.
A typical salt screen will involve the following:
- Determine the desired properties for development and select the appropriate counterions
- Evaluate the physical stability of the salts
- Study the physical and chemical attributes of one or two leading candidates and select one for development
A salt is formed by reaction of an acid with a base. Different types of evaporation and crystallization techniques are most commonly used to prepare various salts of the compound of interest. In addition, a wide variety of solvents with attributes conducive to the compound and the counterions can be manipulated to produce salts.
Once the salt has been prepared, various analytical techniques are then used to determine if the salt has actually been formed. These may include NMR, X-ray powder diffraction, thermal analysis, microscopy, and spectroscopy. Typically, two of these orthogonal techniques are required to confirm formation of the salt and others may also contribute to describe its physicochemical properties.
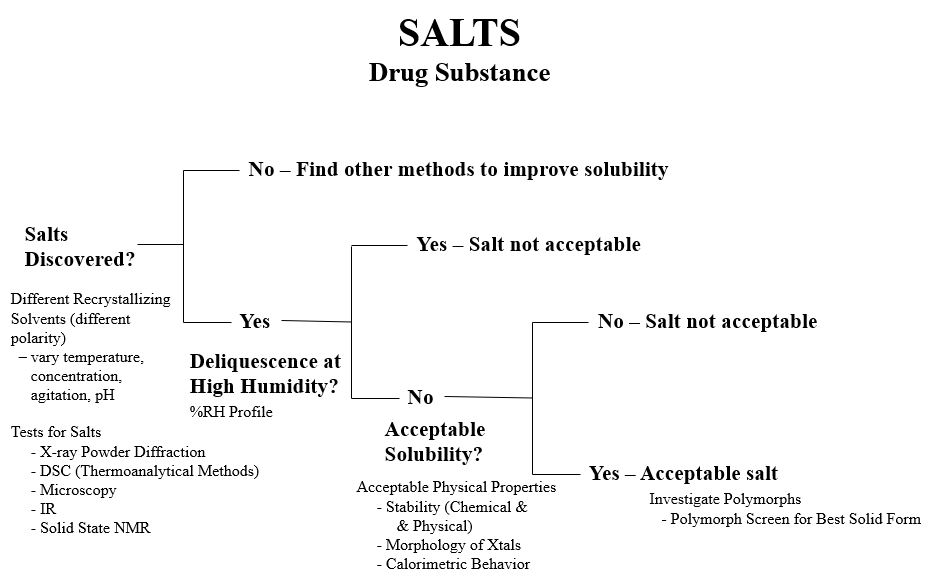
There is a multiple-tier strategy for salt selection targeted for oral formulation (Serajuddin ATM, Pudipeddi M. Salt-Selection Strategies in Handbook of Pharmaceutical Salts (2nd Edition) Weinheim, Germany: Wiley- VCH; 2011).
- Prepare and crystallize all possible salt forms, evaluate crystallinity and aqueous solubility, and select salts with acceptable crystallinity and solubility,
- Evaluate thermal properties and hygroscopicity and choose several for tier 3 evaluation,
- Study physical stability regarding formation of polymorphs, pseudopolymorphs, or disproportionation, choose 2-3 for tier 4, and
- Evaluate bioavailability, chemical stability, compatibility, processability, and choose one for development and select one as backup.
A decision tree based on this strategy is shown in the figure.
The founders of Improved Pharma have been at the forefront of pharmaceutical salt screening activities and continue to be experts in the field. In addition to years of experience, Improved Pharma scientists also utilize high-end techniques such as Raman microscopy and/or IR microspectroscopy as a first pass screen when solids are obtained, and synchrotron X-ray analysis to ensure phase purity or to index patterns.
In summary, selection of a salt form has to be tailored to its intended use, and a balanced approach must be taken with consideration of various properties such as solubility, hygroscopicity, crystallinity, physical stability, chemical stability, and processability.
To learn more about Improved Pharma’s approach to polymorph and salt screening, please refer to this presentation.