WEST LAFAYETTE, Ind., Jan. 26, 2024 /PRNewswire-PRWeb/ — Research for low-dose therapeutics is a growing trend as promising new highly potent therapeutic agents are developed. 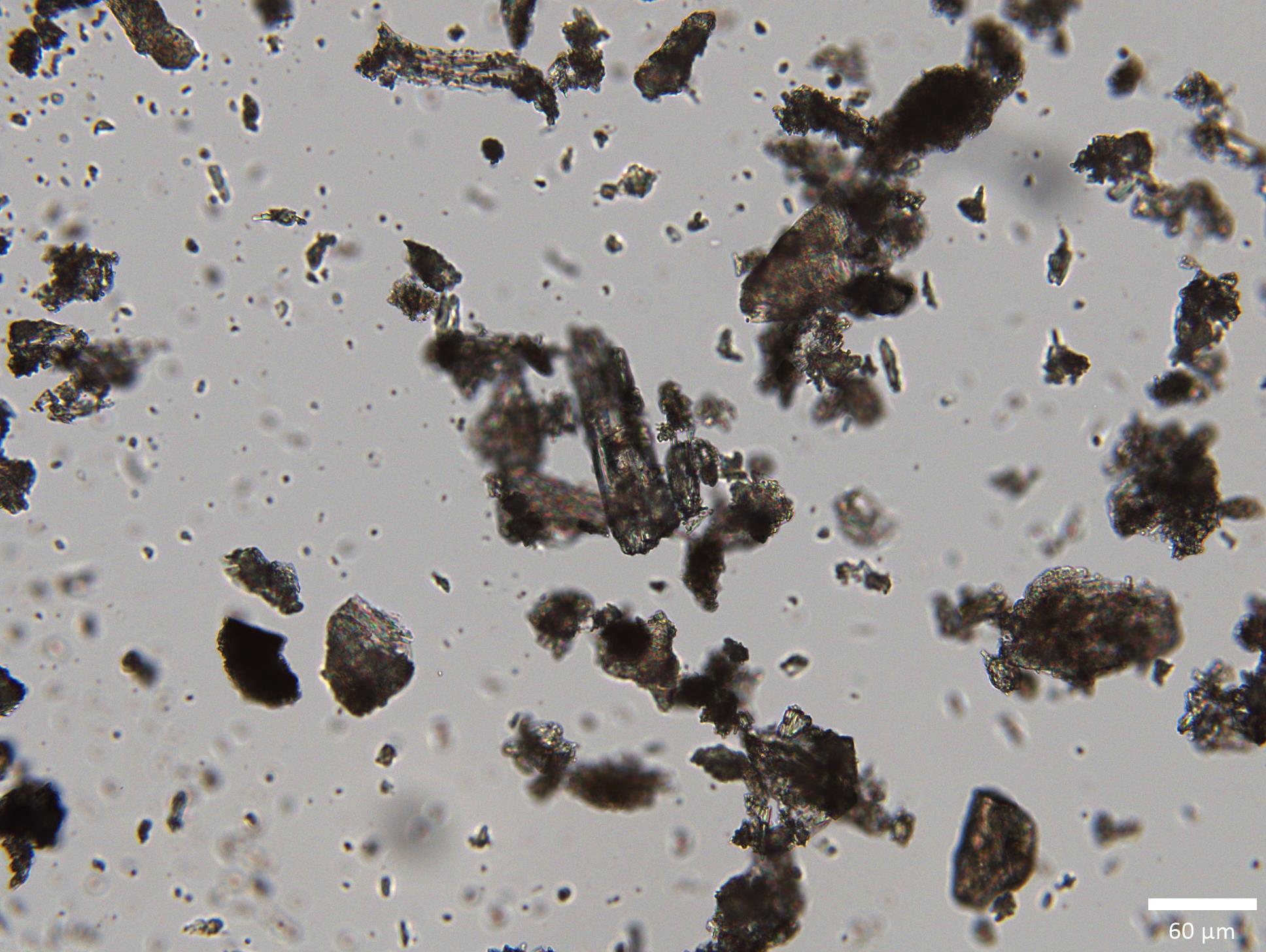 Compounds deemed “highly potent” in pharmaceutical pipelines today describe nearly half of all market approved products and half of the small molecules currently advancing in clinical studies. Therefore, understanding how to achieve blend and content uniformity during powder mixing steps is critical in the design of robust low-dose solid dosage drug products, and yet remains a challenge for conventional blending technologies to reliably achieve this essential critical quality attribute.
Compounds deemed “highly potent” in pharmaceutical pipelines today describe nearly half of all market approved products and half of the small molecules currently advancing in clinical studies. Therefore, understanding how to achieve blend and content uniformity during powder mixing steps is critical in the design of robust low-dose solid dosage drug products, and yet remains a challenge for conventional blending technologies to reliably achieve this essential critical quality attribute.
Through funding provided by Quotient Sciences’ Horizons Initiative, Quotient Sciences researchers, including Dr. David Engers now with Videra Advisors, LLC, and Dr. Dale Purcell of Chemical Microscopy, LLC, consultant to Improved Pharma, initiated a scientific project using resonant acoustic mixing (RAM) technology to investigate RAM as a superior approach to achieve blend uniformity at low concentrations for bulk powders comprised of particles with challenging morphologies.
Using polarized light microscopy (PLM), particle size analysis, and powder flow attribute characterization techniques, with uniformity assessed by high-performance liquid chromatography (HPLC), this work investigated the mixing of caffeine as a model system having particle attributes (acicular particles with blades and agglomerates) that become increasingly important with greater dilution in an excipient matrix. Upon optimization, the results demonstrated that RAM was able to achieve homogeneous mixtures of caffeine in ultra low-dose dilutions, single-digit microgram drug amounts. This work is published in a recent special issue of Pharmaceutical Research dedicated to Professor Kenneth R. Morris.
Their work has contributed to the growing need for technologies that overcome often tedious multi-step laboratory ordered mixing procedures at low concentrations, such as those required to prepare samples of formulation prototypes and for development of analytical methods. Improved understanding of particle physical properties and defining critical process parameters for blending steps at small scale using RAM technology offers the potential for material sparing approaches for seamless scale-up of batch blending processes, achieving uniformity end points and potential time-savings, with potential direct application to advances in continuous manufacturing processes.
TO READ ENTIRE PRWEB PRESS RELEASE, PLEASE CLICK HERE
About Chemical Microscopy
Chemical Microscopy is a materials research and analysis company specializing in the identification, classification, and characterization of small quantities, unknown or foreign substances, using light microscopy, electron microscopy, microspectroscopy, and microchemistry in the pharmaceutical and other industries. The company was founded in 2011.
About Videra Advisors
Videra Advisors is an independent consulting firm that brings development expertise to pre-clinical and clinical stage pharmaceutical and biopharmaceutical companies. Leveraging over 25 years working for sponsor and contract services companies, extensive experience in operations leadership, strategy and enterprise risk management, technical diligence, and scientific-based design of pharmaceutical dosage forms intended for preclinical, clinical development, and commercialization. The company was founded in 2024.
About Improved Pharma
Improved Pharma is a research and information company dedicated to improving pharmaceutical methods, formulations, and processes. Services include solid-state form studies, formulation design, synchrotron techniques, analytical testing, and expert consulting for the development and defense of intellectual property matters. The company was founded in 2006 by Stephen and Sarah Byrn, who also founded SSCI.
