The solubility of drugs in various solvents, particularly water, is a critical physical property that affects their stability, bioavailability, and therapeutic activity in various drug products. Solubility values are generally taken as the amount of drug in solution in equilibrium with a solid, and therefore is a measure of the thermodynamic potential for a drug to dissolve under a given set of conditions. Dissolution describes the rate at which a drug will dissolve under a given set of conditions. In vitro dissolution tests play a crucial role in guiding formulation development and optimization. Improved Pharma offers both solubility and dissolution studies.
Improved Pharma’s dissolution instrumentation includes a Vankel dissolution system with baskets (Apparatus 1), paddles (Apparatus 2), and the Wood’s apparatus (for intrinsic dissolution studies). Detection capabilities include HPLC (Agilent 1100) and UV-Vis (S. I. Photonics model 440 spectrophotometer).
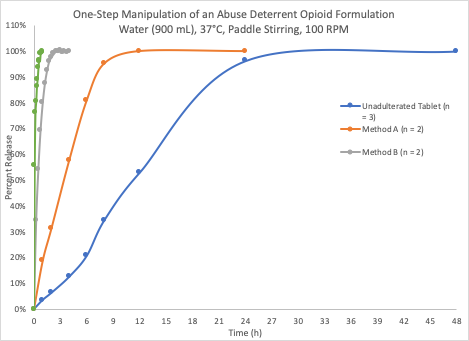
The figure displayed presents UV-Vis data collected on dissolution experiments for various alterations of a drug formulation under development. To learn more about how dissolution studies aid in formulation development, please view this white paper which explains the data presented in the figure.