Not according to the United States Court of Appeals, citing Improved Pharma’s CSO Steve Byrn’s early paper on polymorph screening.
The United States Court of Appeals for the Federal Circuit is known as the Supreme Court of patents, and its rulings are typically considered as significant landmarks in the patent and innovation field. In a case tried in New Jersey of Grünenthal GMBH and Assertio Therapeutics v. Alkem Laboratories, Hikma Pharmaceuticals, and Actavis, the attorneys for Alkem, Hikma, and Actavis argued that the discovery of a new polymorph (polymorph A) of tapentadol was obvious based on the 1995 paper of Byrn, Pfeiffer, Ganey, Poochican, and Hoiberg (Stephen Byrn et al., Pharmaceutical Solids: A Strategic Approach to Regulatory Considerations, 12 Pharmaceutical Res. 945 (1995)). The District Court then ruled against the defendants and said Byrn’s article did not render the discovery of the new polymorph obvious. Put another way, the District Court ruled that the Byrn paper did not show that a person of skill would have a reasonable expectation of success to find polymorph A. The Federal Circuit heard the appeal of Alkem and Hikma that the District Court erred in their ruling; however, the Federal Circuit agreed with the District Court. There was not a reasonable expectation of success that a person of skill would find the new form following the Byrn paper (https://foiadocuments.uspto.gov/federal/17-1153_1.pdf).
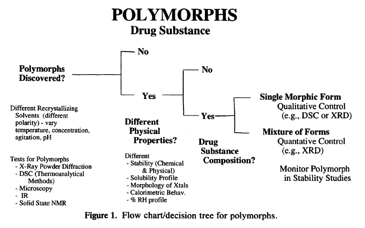 This paper is described in detail below. It describes a crystallization screen involving eight solvents along with many conditions and shows the flow chart shown in the insert. The Federal Circuit stated, “The Byrn article presents a flow chart that outlines a number of variables that may be adjusted during the recrystallization process to determine whether polymorphism occurs in a compound.” That is, this article describes a polymorph screen. The issue was: does this paper and flow chart provide a person of skill with a reasonable expectation of success that they would find the new polymorph? The Federal Circuit noted the following aspects of the Byrn paper:
This paper is described in detail below. It describes a crystallization screen involving eight solvents along with many conditions and shows the flow chart shown in the insert. The Federal Circuit stated, “The Byrn article presents a flow chart that outlines a number of variables that may be adjusted during the recrystallization process to determine whether polymorphism occurs in a compound.” That is, this article describes a polymorph screen. The issue was: does this paper and flow chart provide a person of skill with a reasonable expectation of success that they would find the new polymorph? The Federal Circuit noted the following aspects of the Byrn paper:
- “Byrn does not disclose when it would be appropriate to use particular solvents or a particular mixture of solvents for recrystallization.”
- “Byrn does not outline a particular method to definitively test for polymorphism. Instead, it provides a decision tree outlining, among other things, different ways to gain additional information about whether polymorphs exist for a particular chemical compound and lists various analytical tests to identify polymorphs.”
- “Byrn also instructs a person of skill to “vary temperature, concentration, agitation, pH.” In addition, Professor Joel Bernstein, Cross-Appellants’ expert, testified that when it comes to solution recrystallization “there’s a huge variety of solvents with temperatures, whether you stir or not, and . . . the crystallization is generally carried out by cooling. So the cooling rate can be a major factor in determining what you get.”
- “But Byrn does not provide guidelines regarding which temperature, concentration, agitation, or pH levels are likely to result in polymorphs of particular compounds.”
The Federal Circuit concluded that the lack of disclosure in the Byrn paper supports the testimony of Professor Joel Bernstein that a person of skill would have to manipulate the variables “to determine what the crystal landscape looks like” because “you don’t know what the result is going to be.” Indeed a person of skill could alter any number of variables and still fail to find a polymorph of a particular compound. Thus, the Federal Circuit affirmed that the discovery of the new form was not obvious based on the Byrn paper. One commentary said that because of this finding, ANDA filers have even more incentive to use alternative forms (rather than attempt to overturn patents based on obviousness).
Background
Professors David Curtin and Iain Paul at Illinois, along with Professor Peggy Etter at Minnesota, began working on solid-state chemistry in the late 1960s. Two of their students, Steve Byrn and Joel Bernstein, also started working on solid-state phenomena at that time. Polymorphism was an aspect of this work. Several of the compounds investigated were discovered by Professor David Curtin, who read  the original German writings of Friedrich Konrad Beilstein. Roger Adams of the University of Illinois. saved Beilstein’s writings from destruction when the allies entered Germany right after WWII. Professor Curtin had the entire set of Beilstein above his desk in the Roger Adams laboratory building at Illinois. One of the compounds described in Beilstein was dimethyl 3,6-dichloro-2,5-dihydroxyterephthalate, which the great German chemist Albert Hantszch had studied in the early 1900s. He observed yellow and white forms and thought that there was a structural change when he converted one to the other by crystallization. Steve Byrn, Improved Pharma CSO, worked on that compound as a graduate student and so his interest in color polymorphs began. The insert shows the thermal conversion of the yellow polymorph to the white polymorph on a hot stage microscope. Curtin, Paul, Etter, Byrn, and Bernstein all knew polymorphism was unpredictable and not obvious based on laboratory work and experiments. It only took the Federal Circuit 50 years to concur!
the original German writings of Friedrich Konrad Beilstein. Roger Adams of the University of Illinois. saved Beilstein’s writings from destruction when the allies entered Germany right after WWII. Professor Curtin had the entire set of Beilstein above his desk in the Roger Adams laboratory building at Illinois. One of the compounds described in Beilstein was dimethyl 3,6-dichloro-2,5-dihydroxyterephthalate, which the great German chemist Albert Hantszch had studied in the early 1900s. He observed yellow and white forms and thought that there was a structural change when he converted one to the other by crystallization. Steve Byrn, Improved Pharma CSO, worked on that compound as a graduate student and so his interest in color polymorphs began. The insert shows the thermal conversion of the yellow polymorph to the white polymorph on a hot stage microscope. Curtin, Paul, Etter, Byrn, and Bernstein all knew polymorphism was unpredictable and not obvious based on laboratory work and experiments. It only took the Federal Circuit 50 years to concur!
To further illustrate the complexity of polymorphism, Joel Bernstein and Jack Dunitz wrote their famous paper on Disappearing Polymorphs in 1995, and Steve Byrn’s group at Purdue took a compound given to them by Eli Lilly and Company, 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile, called ROY because it exists in red, orange and yellow forms, and discovered the first three forms of ROY (G.A. Stephenson, T.B. Borchardt, S.R. Byrn, J. Bowyer, C.A. Bunnel, S.V. Snorek, and L. Yu, J. Pharm. Sci, 84(11), 1385, (1995). Later, at least eight more forms of ROY were discovered by Lian Yu at Wisconsin and Eli Lilly and Company (Chen S, Guzei IA, Yu L. New polymorphs of ROY and new record for coexisting polymorphs of solved structures. J Am Chem Soc 2005;127(27):9881-9885.. The same year, 1995, as outlined above, Byrn, Pfeifer, Ganiey, Poochican, and Hoiberg wrote the 1995 paper that was addressed by the Federal Circuit. There was never a belief that the methods outlined would find all the forms or render polymorphs obvious. In fact, whenever lectures addressed polymorphism, the speaker quoted Walter McCrone, who said: “the number of polymorphs found is determined by the time, money and effort spent finding them.”
In that same timeframe, the early 1990s, Steve Byrn and his good friend Ralph Pfeiffer began teaching short courses on polymorphism, first in Bradford, England, where Peter York and David Grant joined them, and then at Purdue University. In 1991, Steve Byrn and his wife Sally founded SSCI as a research and information company and started teaching short courses around the US. FDA scientists Drs. Ganey, Poochician, and Hoiberg attended some of the first courses, and that’s how the 1995 paper was developed. Mike Ganey was especially crucial in developing the flow charts. SSCI’s short course was in high demand, and SSCI has taught more than 100 short courses on the solid-state chemistry of drugs. Also, in 1993 SSCI opened its laboratories and started doing polymorph screens. SSCI advertised these screens at the AAPS meetings, and this is how polymorph screens became important.
In conclusion, it is now clear that scientists and the US Districts and Federal courts agree that polymorphism is unpredictable and not obvious despite the general statements in the Byrn paper. Further, it is clear from the vast experience of Improved Pharma that identifying and controlling the polymorph is a key to successfully developing a new drug and protecting intellectual property.
