The goal of polymorph screening is to try to find all possible crystal forms by small-scale crystallization and to select a stable form that can be manufactured and formulated into a dosage form. Polymorph screening consists of several key steps.
- Discover all possible polymorphs. How many can be identified?
- Evaluate the stability order for those forms. What is the thermodynamically stable polymorph at room temperature? If enantiotropic pairs exist, what are the transition temperatures?
- Study the physical and chemical attributes of one or two leading candidates and select one for development
Crystallization, evaporation, precipitation, slurrying, and melt crystallization are widely used to discover polymorphic forms. Regarding crystallization, we can choose various solvents, solvent mixtures, cooling rate, or supersaturation level. Quick cooling and precipitation usually result in metastable forms. Melt crystallization may help discover polymorphs not observed in solution. Crystallization in solvents sometimes produces solvates instead of polymorphs. Slurrying in different solvents is a popular approach used to isolate stable polymorphs. It is based on solution-mediated transformation. In such cases a metastable form is dissolved, and hopefully, a more stable form will grow in the expense of the metastable form.
A number of techniques are available for identifying solid forms of a compound. These techniques include X-ray powder diffraction, thermal analysis, microscopy, spectroscopy, and solid-state NMR. Usually, a combined use of the above techniques is necessary to provide a full understanding of each polymorph.
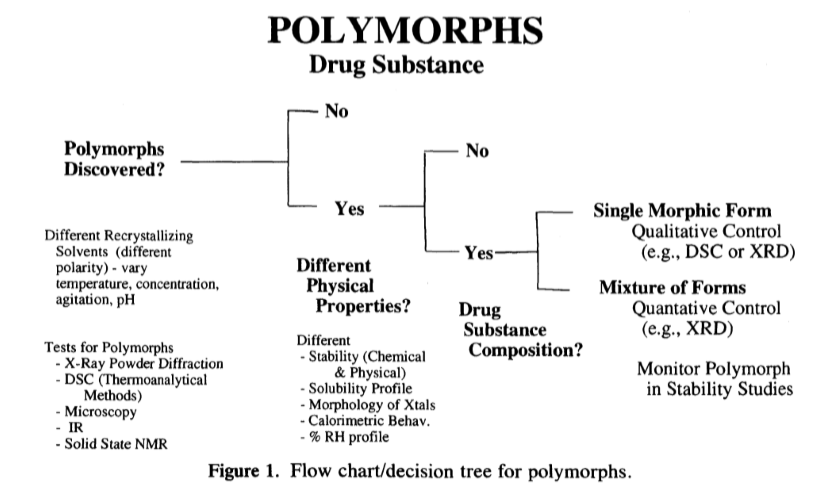
Improved Pharma experts have been involved in pharmaceutical polymorph screening since the beginning. In 1995, Dr. Byrn published a paper with the FDA using decision trees to describe a strategy to identify the best solid form early in development [1]. The decision tree for polymorphs is shown in Figure 1 and was the precedent of decisions trees later included in ICH guidances that are still used today. Recently, the United States Court of Appeals for the Federal Circuit cited Dr. Byrn’s landmark paper on polymorph screening. They concluded that polymorphism is unpredictable and not obvious based on laboratory work and experiments. Improved Pharma scientists fully understand the importance of tailoring polymorph screens for each compound to ensure a successful outcome. There is no such thing as a “routine polymorph screen”, and providers who offer such services are building unnecessary risks into their clients’ products. Furthermore, Improved Pharma also offers a Synchrotron-based Polymorph Screen (SPS). An SPS is only slightly more expensive than a tailored screen and is especially useful for complicated systems where there are mixtures of polymorphs.
To learn more about Improved Pharma’s approach to polymorph and salt screening, please refer to this presentation.
- Byrn S, Pfeiffer R, Ganey M, Hoiberg C, Poochikian G. Pharmaceutical solids: a strategic approach to regulatory considerations. Pharm Res 1995; 12: 945-954.