In the area of oral product development, judicious use of several methods can accelerate development and reduce costs. A critical early step involves considering 3 important issues:
- The structure of the molecule
- The solubility of the molecule in water
- The X-ray powder diffraction pattern of the substance for development
The structure allows rapid determination of the feasibility of salt formation and a general prediction of stability. The feasibility of salt formation is determined by the presence of acidic or basic groups and the estimated pK of these groups from analogous molecules and literature references. The solubility of the molecule can be rapidly determined on small amounts of material using microscopic or small-scale techniques. Finally, small amounts of material can also be used to determine the X-ray powder diffraction pattern, allowing the determination of the crystallinity of the material.
Most molecules under development are poorly soluble. Assuming poor solubility the following approaches can be taken to accelerate the development of a toxicology formulation and at the same time develop important information for a human formulation for clinical trial. If the molecule can form a salt either HCl or NaOH is used to salify the molecules and the solubility is reestimated. If acceptable solubility is achieved, these simple salts can be used for quick advancement into animal studies. Meanwhile, more extensive salt studies can be performed to either confirm the viability of the original hydrochloride or sodium salt or develop an improved salt for clinical trials.
If acceptable solubility of the salt is not achieved or the molecule does not form a salt then the neutral molecule must be further formulated to achieve high solubility and high animal exposure. Perhaps the best alternative at this stage is an amorphous solid dispersion as has been extensively discussed in the literature. Anane-Adjei provides a particularly interesting review of the approach Astra-Zeneca uses at this point to achieve high blood levels of candidates in animals.(Anane-Adjei et al., 2022). At this stage, a simple amorphous screen is generally recommended. Of particular concern is whether the amorphous formulation resists crystallization in the suspensions typically used for toxicology. To address this concern, a simple crystallization screen is recommended. Although more elaborate screens and decision trees are possible, it is often possible to circumvent these systems by performing a few simple experiments in some cases microscopically.
Once the formulation for animal toxicology is developed some time is available in the accelerated timeframe to develop backup toxicology formulations and the clinical formulation for the IND. Several strategies have been utilized to accelerate drug development and reach the IND rapidly. In 1995 Colin Gardner of Merck introduced a flow chart showing the API synthesis and development of clinical supplies for the first in human trials in one year. In this early flow chart, drug substance synthesis and process development were carried out parallel to preformulation/formulation design/development and safety studies. Despite the early introduction of the concept that an IND can be submitted in one year, it has been difficult to achieve this goal except in very favorable cases. One of the difficulties is the availability of API, and another one of the difficulties is accelerating toxicology studies.
In 2007, SSCI, which was at that time owned by Aptuit, introduced their IND-I-GO (INDIGO) program offering fast development in a CRO environment. INDIGO was tailored to working with BCS Class II compounds and poorly soluble compounds. This INDIGO offering was supported by an example case study on the poorly soluble drug itraconazole and was summarized in a 2010 publication (Engers et al., 2010). Additionally, Byrn, Zografi, and Chen, and Byrn and Henck outlined strategies based on solid state chemistry for reducing development time (Byrn & Henck, 2007; Byrn et al., 2011). These publications contained much more detail on how to carry out screens and are discussed in more detail below. In this same timeframe, Chorus, a Lilly-based firm focused on fast development, introduced their strategy for accelerated development to Phase II (Longman, 2007). In this early publication, they suggested that it was possible to develop a compound to Phase II in 30 months for $3 million dollars in contrast to the industry average of 42 months and $30 million dollars. The Chorus approach involves a virtual company that heavily uses preferred CROs. Chorus has been quite successful and Lilly has now established Chorus as an independent entity. Figure 1 shows a detailed 52-week strategy.
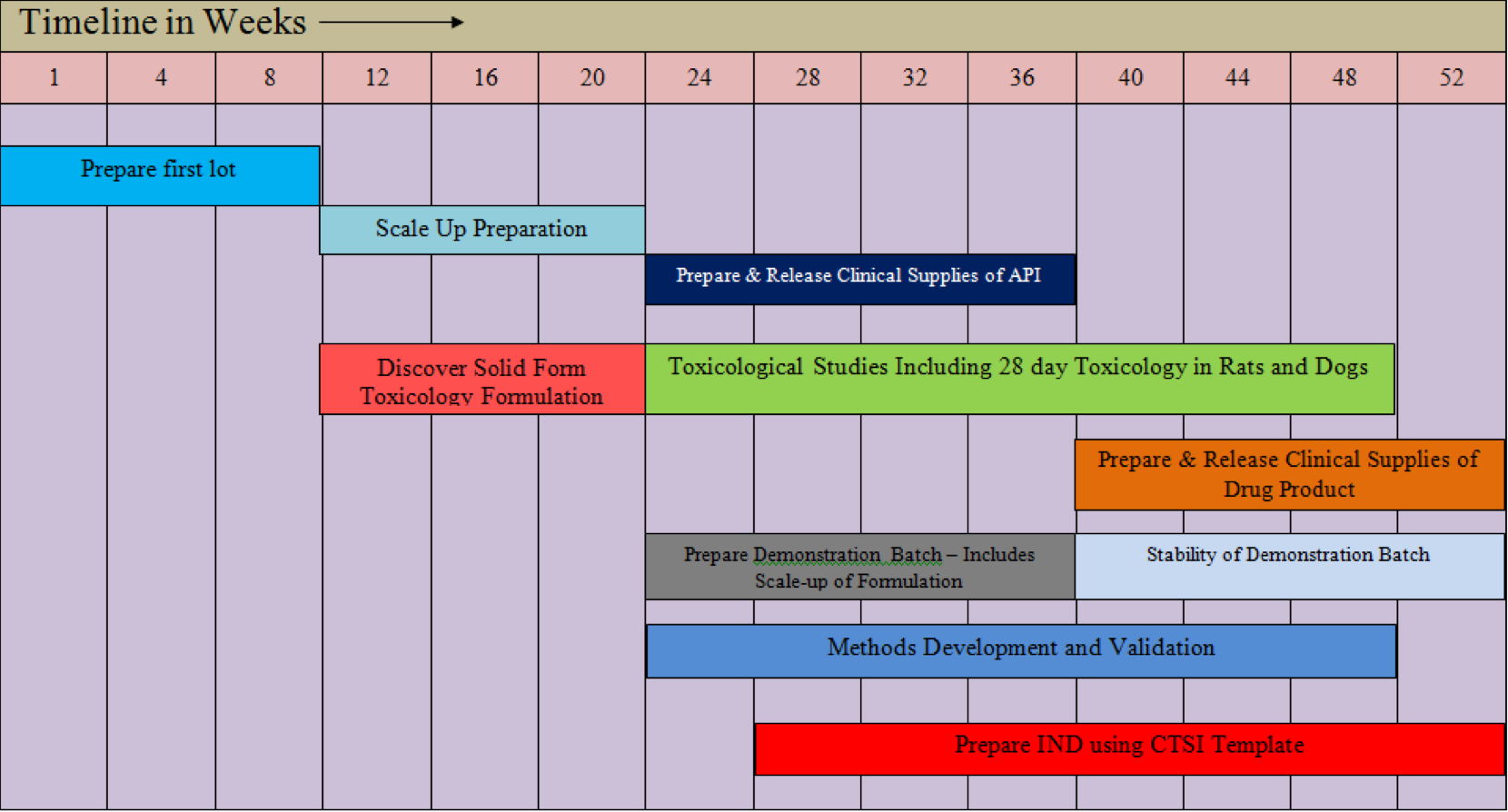
Figure 1. Detailed 52 week strategy for accelerated drug development.
The top three bars show the synthesis of the API. Depending on dose, several kilograms of API will be required for toxicology studies which are shown in the fourth bar. A key aspect of the toxicology study is the determination of the toxicology formulation. As pointed out by the Merck group, the toxicology formulation can be critical for poorly soluble drugs. If not enough of the drug can be dissolved then it is impossible to advance that lead (Palucki et al., 2010).The bottom 4 bars outline the formulation and manufacture of clinical supplies and the regulatory activities needed to file an IND. Engers, Byrn, Newman and coworkers were able to meet the timelines in figure 9 and develop a mock IND for itraconazole (Engers et al., 2010).
In conclusion, one of the goals of accelerated development is to eliminate compounds that are clinically unacceptable or cannot be developed early so that more resources are available to focus on compounds that are truly acceptable. For example, if a material is so insoluble its MAD or MTD cannot be determined in animal studies then this must be determined as early as possible. Likewise if a compound has unacceptable neurotoxicity this needs to be determined as early as possible. Thus, a strategy, like that outlined above, that focuses on the most soluble formulation early will provide important information in both of these cases.
To learn more about Improved Pharma’s approach to accelerated development, please visit Accelerated Formulation Development and Synchrotron-Based Fast to IND (S-fIND) | Improved Pharma.
References
Anane-Adjei, A. B., Jacobs, E., Nash, S. C., Askin, S., Soundararajan, R., Kyobula, M., Booth, J., & Campbell, A. (2022). Amorphous solid dispersions: Utilization and challenges in preclinical drug development within AstraZeneca. International Journal of Pharmaceutics, 614, 121387.
Byrn, S., & Henck, J. O. (2007). Molecular Delivery System in Preclinical Timeframe. Drug Discovery Today, 12, 189-196.
Byrn, S., Zografi, G., & Chen, X. (2011). Accelerating Proof of Concept for Small Molecule Drugs Using Solid-State Chemistry. J. Pharm. Sci., 99, 3665-3675.
Engers, D., Jimenez-Novoa, J., Gent, P., Jossack, S., Campbell, C., Thompson, J., Ivansevec, I., Templeton, A., Byrn, S., & Newman, A. a. (2010). A solid state approach to enable early development of compounds: Selection and animal bioavailability studies of an Itraconazole Solid Dispersion. J. Pharm. Sci., 99, , 3901-3922.
Longman, R. (2007). Lilly’s Chorus Experiment. In-Vivo, 25, 1-5.
Palucki, M., Higgins, J. D., Kwong, E., & Templeton, A. C. (2010). Strategies at the Interface of Drug Discovery and Development: Early Optimization of the Solid State Phase and Preclinical Toxicology Formulation for Potential Drug Candidates. J. Med. Chem., 53, 5897-5905.
