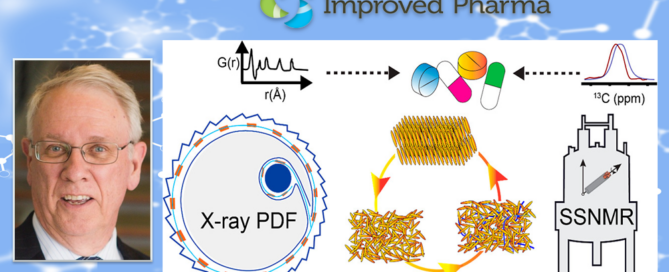
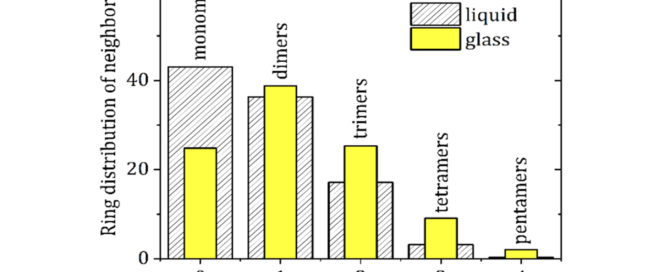
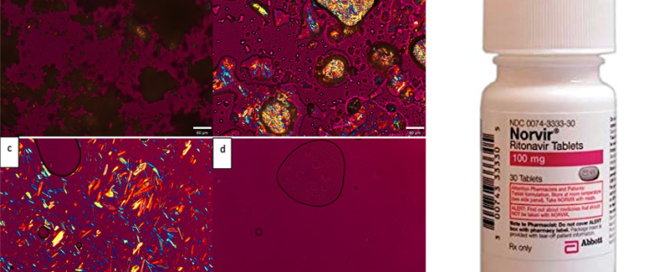
Report on Co-Processing Plenary Lectures at the SPS-XRPD-4 Workshop
The 4th Spring Pharmaceutical Synchrotron X-Ray Powder Diffraction Workshop was held June 10-11, 2024, at the Novartis campus in Basel, Switzerland. The SPS-XRPD workshops are a collaborative effort between Excelsus Structural Solutions in Switzerland and Purdue University, Argonne National Laboratory, and Improved Pharma in the United States. These workshops bring together experts from the Read more