Since the ritonavir crisis in 1998, when a lifesaving HIV protease inhibitor had to be withdrawn from the market for almost a year due to a sudden and unpredictable appearance of a new less soluble polymorph, ritonavir has been of interest to solid state chemists, crystallographers, and drug development scientists.[1-4] After the appearance of Form II ritonavir, two new solvates and an anhydrous form were discovered by Transform Pharmaceuticals.[4] In 2015 a most interesting review highlighted the appearance of Form II as perhaps “the most notorious example of disappearing polymorphs.”[2]
In 1998, ritonavir crystallized in gel caps and failed dissolution. No amount of cleaning could prevent this crystallization. Investigations showed that a new late-appearing polymorph, Form II, had appeared and crystallized out of the gel caps. Further investigation showed that Form II had about half the solubility of the original Form I. Ultimately the Form I gel caps had to be withdrawn from the market and reformulated into a new formulation.[3] The delay and withdrawal cost Abbott Laboratories, now AbbVie, about $900,000,000.
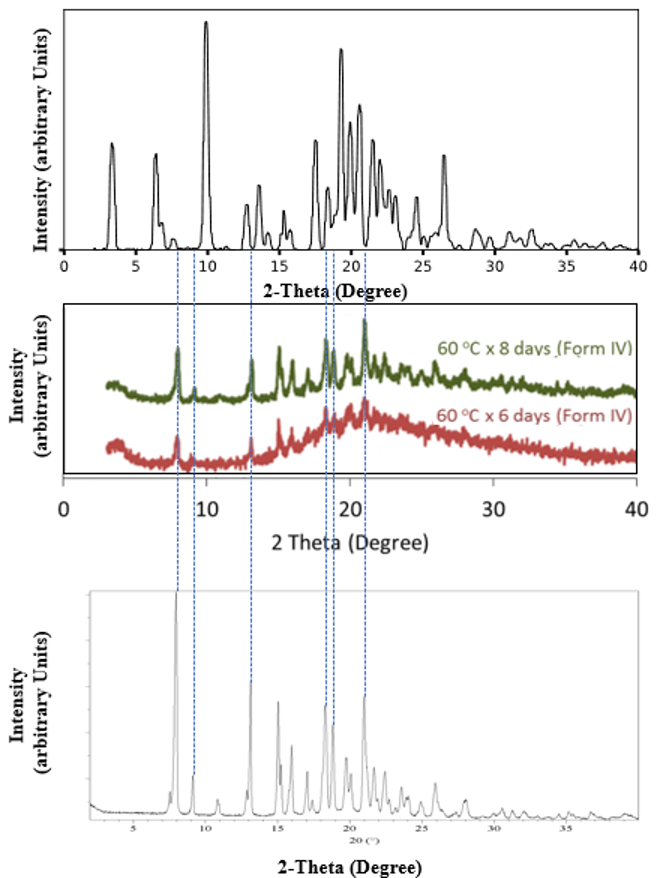
The appearance of Form II was unpredictable. This type of late-appearing polymorph is a feared phenomenon that is generally known among solid state chemists.[1, 2] For ritonavir, Form I was the only known form during the entire drug development process, including clinical trials and launch. Form II appeared more than a year after launch. Five years after the appearance of Form II, a polymorph screen by Transform Pharmaceuticals showed two new solvates and a third anhydrous form, which they named Form IV.[4] Then, almost 20 years later in 2022 two laboratories in the Midwest United States, one at AbbVie and one at Improved Pharma, LLC, almost simultaneously discovered another anhydrous form by melt crystallization. This newer anhydrous form was named Form III.[5, 6] Curiously, in 2015 Kawakami in Japan was studying the melt behavior of ritonavir Form I and reported he had obtained the anhydrous Form IV Transform had reported, but he had actually unknowingly generated Form III.[7] Figure 1 compares the diffraction pattern of Kawakami’s form to the Transform Form IV and Improved Pharma’s Form III. The comparison of these diffraction patterns suggests that Kawakami actually had generated Form III mixed with an amorphous or disordered form.[7]
Figure 1. Comparison of Transform’s Form IV (top), Kawakami “Form IV” (middle), and Improved Pharma’s Form III (bottom).
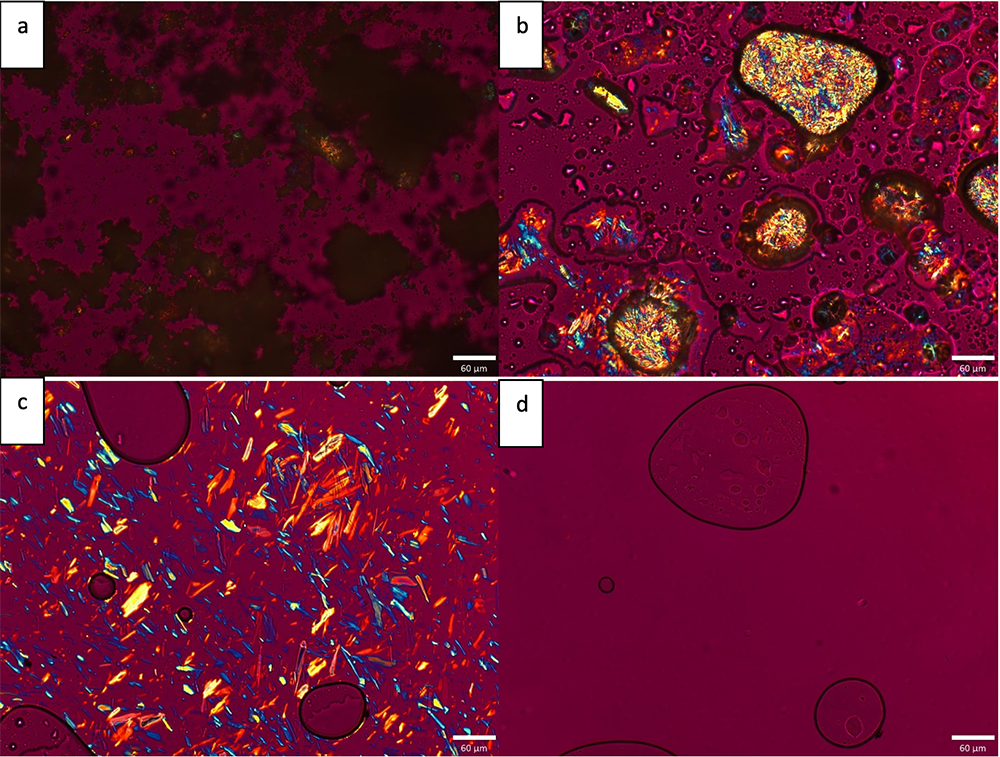
The new Form III was discovered by melt crystallization. This is the same method used by the University of Wisconsin group, headed by Lian Yu to discover new ROY polymorphs. Based on these observations it is clear melt crystallization should be included as a method for polymorph screening if the drug is stable enough to melt. Often DSC can be used to provide an early indication of stability of the melt. Careful hot stage optical microscopy at Improved Pharma via Chemical Microscopy, LLC, showed an onset of melting of Form III of 113.7 ⁰C and a melting point of 117.9 ⁰C (Figure 2). These results conformed to the DSC results.
Figure 2. HSOM images for Form III: a) Melt onset at 113.7˚C; b) continued melting at 115.8 ˚C; c) continued melting at 117.0 ˚C; d) melt complete at 117.9 ˚C
Form III was stable in limited experimental studies at high temperature. Form III is less thermodynamically stable than Form I and Form II from 20 ⁰C to the melting point. Interestingly, Form III showed trans conformations about the methyl urea and carbamate groups whereas Form I showed cis conformation in the methyl urea group and trans conformation in the carbamate, and Form II showed a trans conformation in the methyl urea and a cis conformation in the carbamate group.
In summary, there have been at least three different occurrences of late-appearing polymorphs for ritonavir: the original appearance of Form II, the appearance of the anhydrous form in 2005, and the appearance of Form III in 2022. Even with one of the most studied pharmaceutical compounds, the potential to find new polymorphs is always there. To learn more about polymorphs screening, please visit us at Polymorph Screening Services | Improved Pharma
- Bauer, J.; Spanton, S.; Henry, R.; Quick, J.; Dziki, W.; Porter, W.;Morris, J. Ritonavir: An Extraordinary Example of Conformational Polymorphism. Res. 2001, 18, 859−866.
- Bučar, D. K.; Lancaster, R. W.; Bernstein, J. Disappearing Polymorphs Revisited. Chem. Int. Ed. 2015, 54, 6972−6993.
- Chemburkar, S. R.; Bauer, J.; Deming, K.; Spiwek, H.; Patel, K.;Morris, J.; Henry, R.; Spanton, S.; Dziki, W.; Porter, W.; Quick, J.;Bauer, P.; Donaubauer, J.; Narayanan, B. A.; Soldani, M.; Riley, D.;McFarland, K. Dealing with the Impact of Ritonavir Polymorphs on the Late Stages of Bulk Drug Process Development. Process Res. Dev. 2000, 4, 413−417.
- Morissette, S. L.; Soukasene, S.; Levinson, D.; Cima, M. J.; Almarsson, O. Elucidation of Crystal form Diversity of the HIV Protease Inhibitor Ritonavir by High-throughput Crystallization. Natl. Acad. Sci. U. S. A. 2003, 100, 2180−2184.
- Parent, S.; Smith, P.; Purcell, D.; Smith, D.; Bogdanowich-Knipp, S.; Bhavasar, A.; Chan, L.; Croom, J.; Bauser, H.; McCalip, A.; Byrn, S.; Radocea, A., Ritonavir Form III: Lightning strikes twice at the same time, 137 miles apart. Chem Rxiv 2022, https://doi.org/10.26434/chemrxiv-2022-49tzw.
- Yao, X.; Henry, R. F.; Zhang, G. G. Z. Ritonavir Form III: A New Polymorph After 24 Years. Chem Rxiv 2022, DOI: 10.26434/chemrxiv-2022-35fwp.
- Kawakami, K.; Harada, T.; Miura, K.; Yoshihashi, Y.; Yonemochi, E.; Terada, K.; Moriyama, H. Relationship Between Crystallization Tendencies During Cooling from Melt and Isothermal Storage: Toward a General Understanding of Physical Stability of Pharmaceutical Glasses. Pharmaceutics 2014, 11, 1835−1843.
