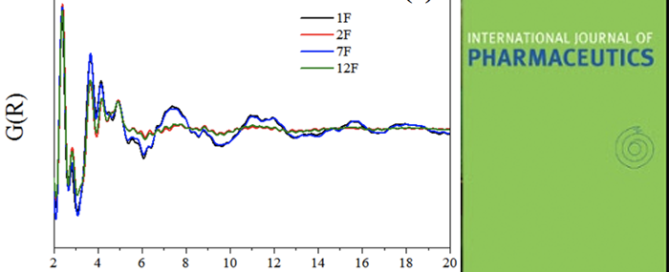
The Structure of Liquid and Glassy Carbamazepine
To enhance the solubility of orally administered pharmaceuticals, liquid capsules or amorphous tablets are often preferred over crystalline drug products. However, little is known regarding the variation in bonding mechanisms between pharmaceutical molecules in their different disordered forms. In this study, liquid and melt-quenched glassy carbamazepine have been studied using high energy X-ray diffraction and Read more