Achieving Control in IND Drug Development: Improved Pharma’s Comprehensive Strategy
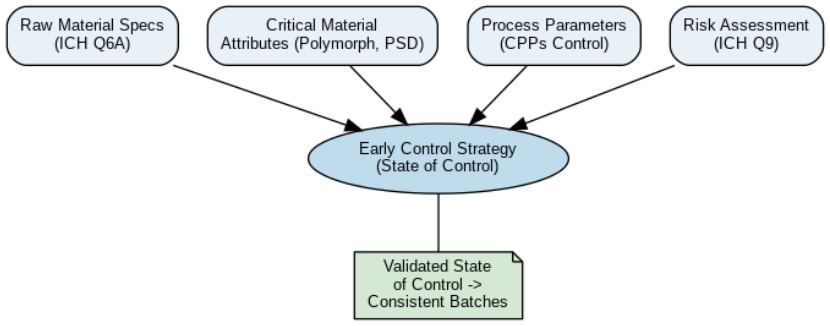
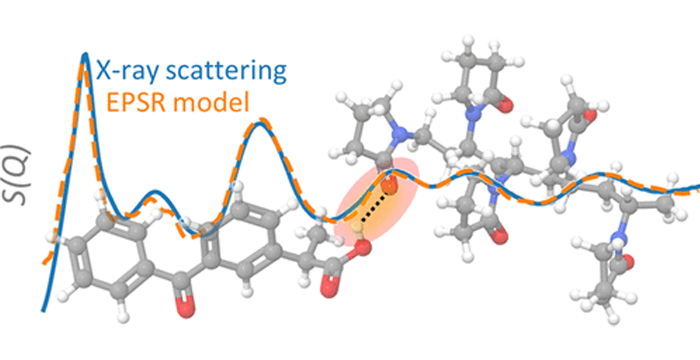
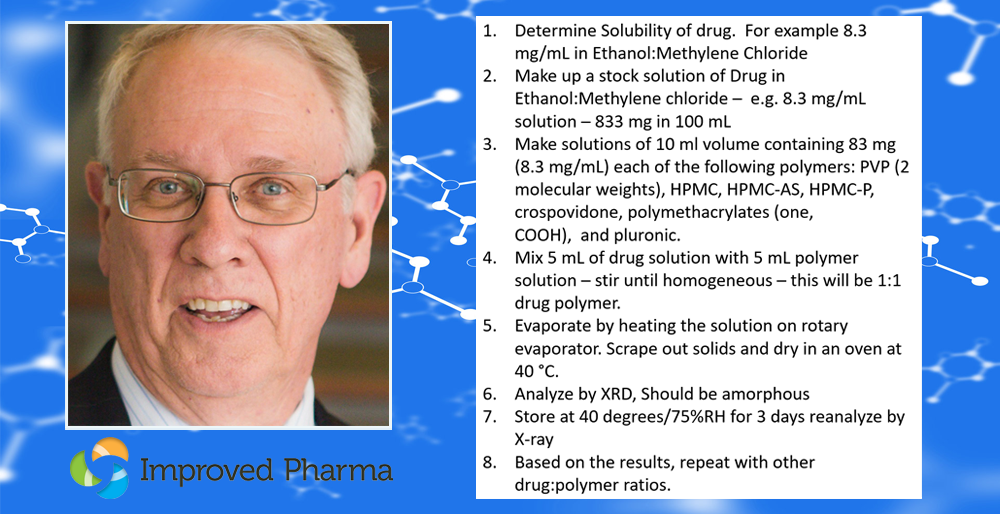
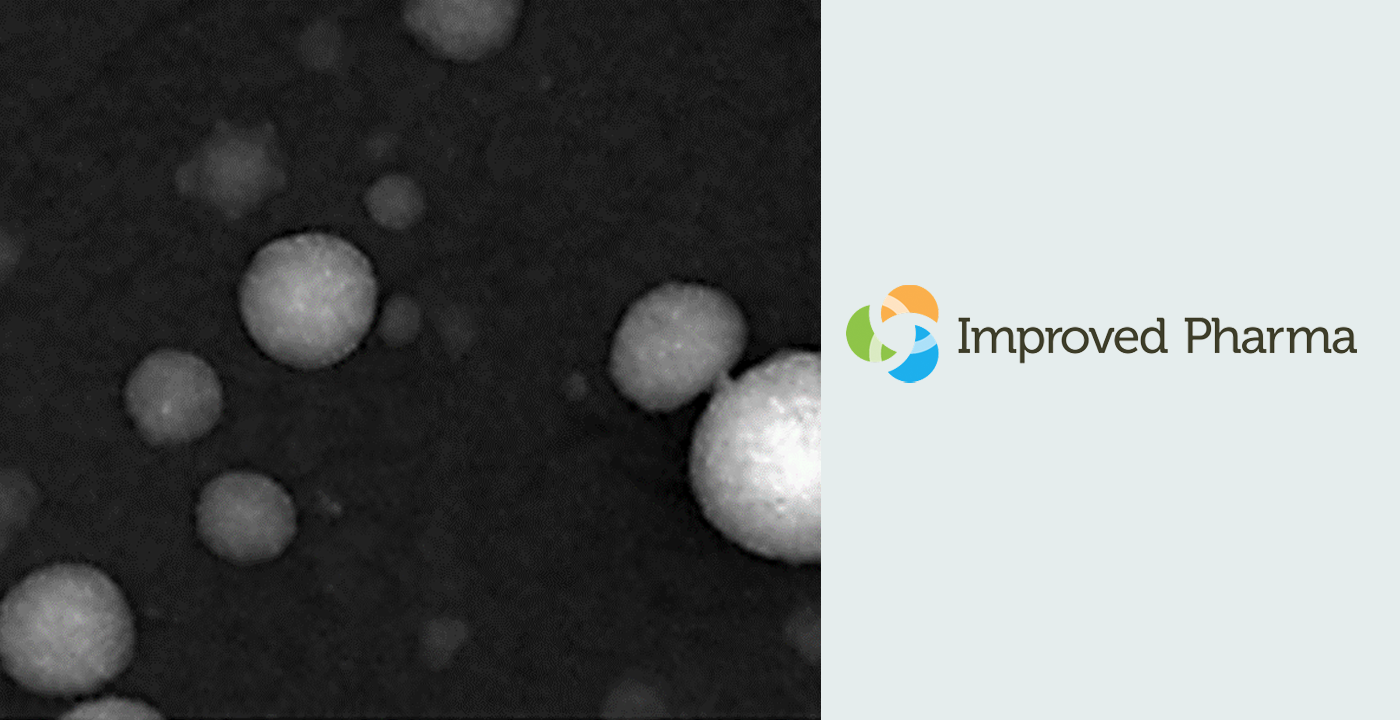
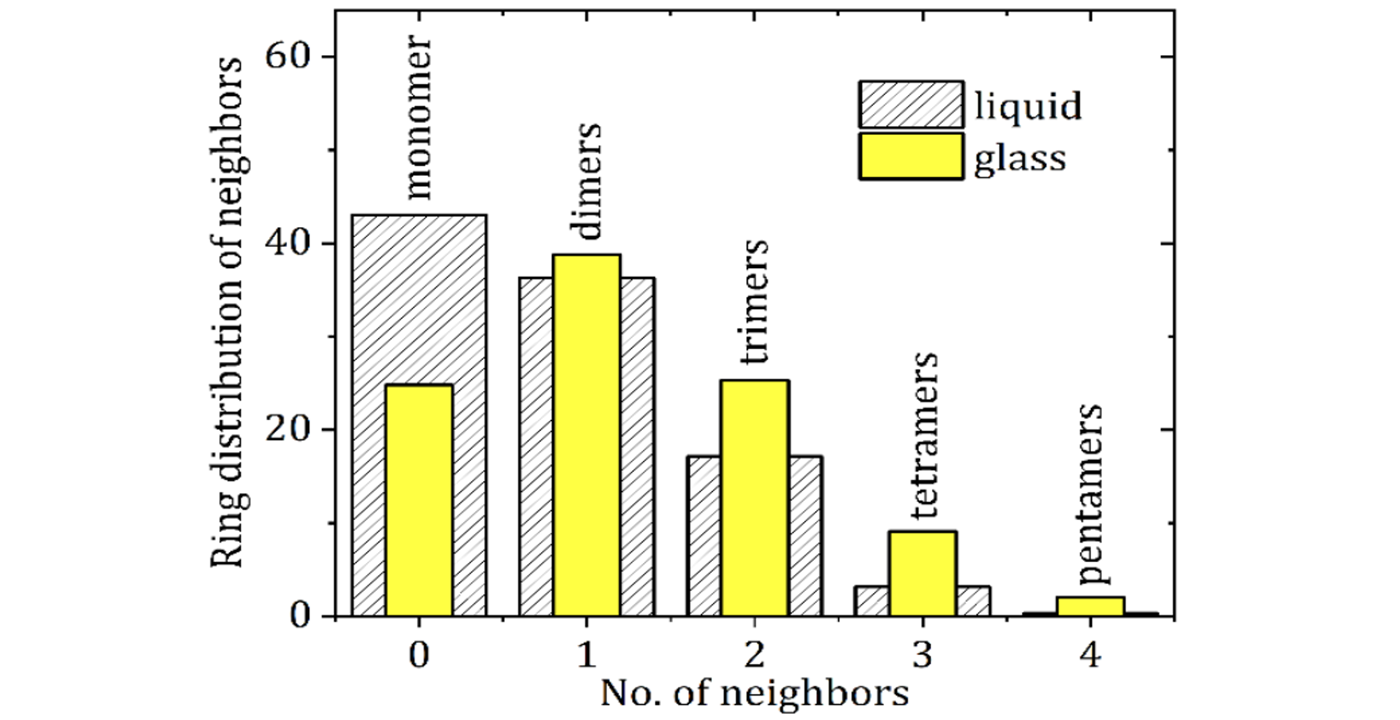
Ed olimpio2025-04-21T18:31:03+00:00Achieving control is essential in early-stage pharmaceutical development, not only to meet regulatory expectations but to ensure consistency, high-quality, and reliability of clinical batches. Improved Pharma has developed a comprehensive control strategy that supports Read more