Authors Jared Smit, David Engers, Steve Byrn
In modern small-molecule development, solid-state characterization is far more than a routine analytical task.(Byrn, Zografi et al. 2025) It defines the manufacturable form of an API, supports stability projections, drives formulation decisions, underpins regulatory submissions, and often determines the strength of a company’s IP portfolio. Yet in today’s global outsourcing economy—where sponsors are under extraordinary pressure to cut costs—critical polymorph, powder X-ray diffraction (PXRD), and solid-state studies are increasingly performed in low-cost laboratories. These environments often operate under business models that treat crystallography as a commodity rather than a scientific discipline requiring precision, orthogonality, and rigorous data governance.
The result of this trend is predictable: misassigned crystal forms, ambiguous PXRD patterns, incomplete polymorph screens, unrecognized minor phases, weak data integrity practices and less than optimal patent grants. These errors are rarely discovered early. They often appear months later in failed stability studies, unexplained batch variability, regulatory questions, or during ligitation of the patents containg the data. And by that time, the cost of repeating the work is far higher than the cost of doing it correctly from the beginning.
Our U.S.-based company takes a fundamentally different approach. We integrate high-performance in-house PXRD, rigorous single-crystal X-ray diffraction (SCXRD), high-resolution synchrotron PXRD and PDF analysis through Argonne National Laboratory with expert level interpretation of the data As such we provide the level of clarity, traceability, and defensibility that today’s CMC, regulatory, and IP landscapes demand.
Misassigned Crystal Forms and Erroneous Crystal Structures
Misassigned crystal forms are not hypothetical. They have been documented in the scientific literature even though scientists rarely publish their known failures.(Taylor and Wood 2019) The technical causes behind these misassignments are well known. Limited PXRD resolution can obscure peak splitting and overlapping reflections. Short counting times inflate noise and hide weak features of the diffractogram. Improperly calibrated instruments shift PXRD peak positions. Over-smoothed diffractograms eliminate subtle but meaningful peaks. Incomplete polymorph screening increases the likelihood that additional polymophs went undiscovered. The absence of orthogonal methods such as DSC, TGA, Raman spectroscopy, and solid-state NMR leaves the interpretation resting on a narrow evidentiary foundation. Perhaps most importantly, laboratories that lack access to high-end spectroscopic particle analysis, SCXRD or synchrotron PXRD must attempt to resolve difficult cases using tools that simply cannot provide the needed clarity. These factors are not isolated errors—they are systemic consequences of analytical environments optimized almost entirely for speed and cost.
Solid-State Chemistry of Modern APIs is Complex
Modern APIs are frequently poorly crystalline, nanocrystalline, disordered, or characterized by extremely subtle polymorphic differences, all of which challenge standard laboratory PXRD instruments.(Byrn, Zografi et al. 2025) This is exactly where Improved Pharma’s expertise and access to Argonne’s Advanced Photon Source becomes decisive. High-resolution synchrotron PXRD provides extremely sharp peak profiles, exceptional signal-to-noise ratios, precise peak positions, and reliable detection of trace phases well below the sensitivity threshold of conventional diffractometers. These capabilities allow for clear separation of overlapping peaks, detection of minor phases, characterization of nanocrystals, and accurate identification of closely related polymorphs. Ambiguity is replaced with certainty, and borderline patterns that would be misinterpreted in a low-cost environment become fully understandable.
A particularly frequent problem in solid-state outsourcing is the incorrect labeling of materials as “amorphous.” Many laboratories use the term simply because a PXRD pattern lacks sharp peaks. But genuine amorphous characterization benefits from a combination of orthogonal techniques, including pair distribution function (PDF) analysis, Raman mapping, and/or polarized light microscopy (PLM). PDF, in turn requires high-energy synchrotron data. PDF analysis reveals short-range order, distinguishes true amorphous systems from poorly crystalline ones, detects nanocrystalline seeds, and can differentiate between multiple amorphous states. This level of structural insight prevents one of the most common and costly sources of misinterpretation in solid-state systems. Other techniques can also detect very low levels of crystalline material. Raman mapping identifies domains of crystalline material, and in PLM, crystallites appear like stars in the night sky. Figures 1 and 2 show excerpts from a poster presented at a special session at the 2025 AAPS meeting illustrating the power of Raman mapping to detect crystallinity in amorphous dispersions.(Smith 2025)
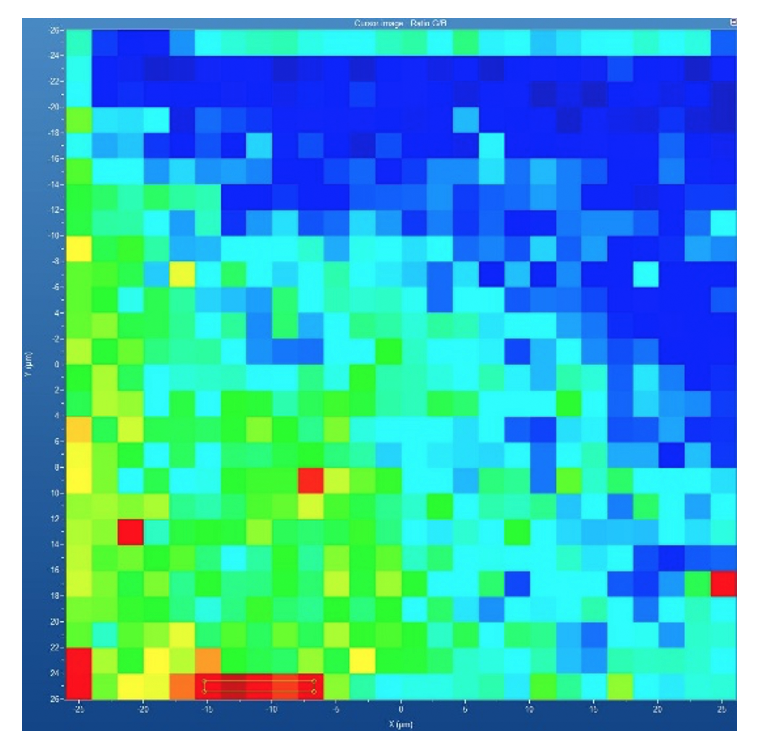
Improved pharma feature 3
Figure 1. Raman mapping profile for crystalline nilotinib character (red) and H-bonded nilotinib (blue)
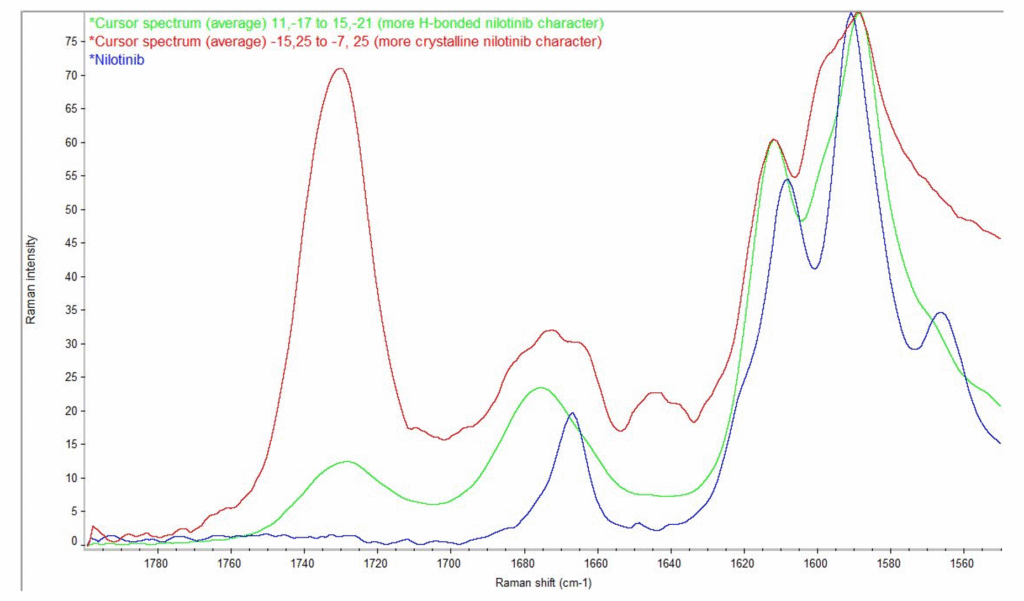
IMproved pharma feature image 2
Figure 2. Raman spectra from map representing more crystalline character (red), more H-bonded character (green), and nilotinib (blue)
High Quality Single Crystal XRD
For crystalline material, single-crystal X-ray diffraction provides the definitive structural fingerprint of a form. High-quality SCXRD yields the full atomic arrangement, including hydrogen bonding networks, solvent or water molecule positions, precise unit cell parameters, and unambiguous space group determination. Once we solve a crystal structure, we generate the corresponding theoretical powder diffraction pattern and compare it to our experimental PXRD patterns obtained on the bulk solids. An example is shown in Figure 3 which compares the calculated PXRD pattern of sodium acetate trihydrate from the single crystal structure with the experimental PXRD pattern of the bulk material. Because the patterns match and no extra peaks are observed in the pattern of the bulk material, we know the bulk material represents a single phase. These techniques may include in-house PXRD and synchrotron PXRD. We may compare the SCXRD calculated patterns to the PXRD patterns obtained from stability samples, manufacturing batches, and samples generated from crystallizations we conducted in-house. When SCXRD and synchrotron data align, the form assignment is beyond dispute.
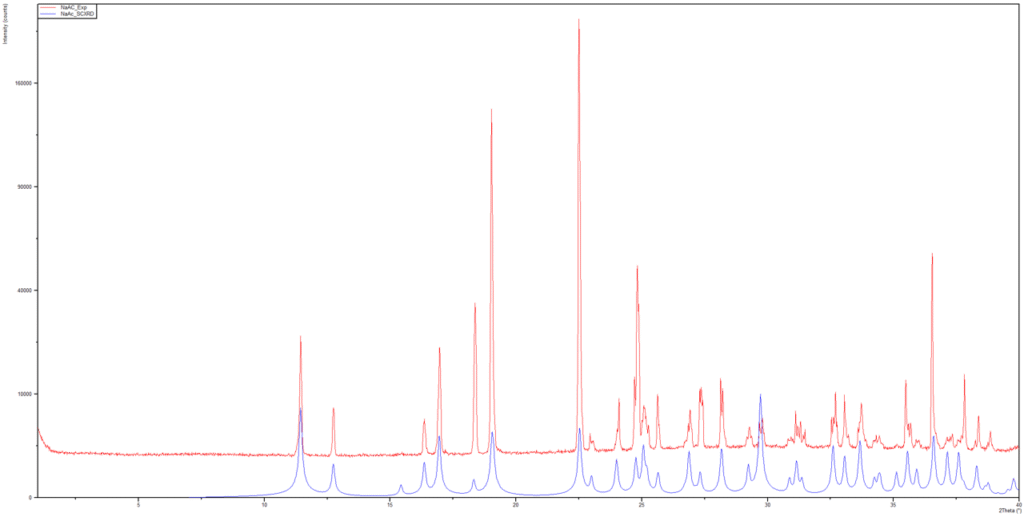
Figure 3. A comparison of the calculated PXRD pattern of sodium acetate trihydrate from the single crystal structure (blue) and the experimental PXRD pattern of the bulk (red)
Patents and Data for Regulatory Submissions in Complex Solid State Environments
This level of structural certainty matters deeply for patents. Crystal-form IP is only as strong as the analytical data supporting it. During prosecution patent examiners may scrutinize peak clarity, reproducibility, impurity detection, unit cell accuracy, and consistency. The combination of SCXRD, synchrotron PXRD, in-house PXRD, PDF analysis, and orthogonal data such as DSC, Raman spectroscopy or SSNMR provides a multi-layer evidence package that strengthens arguments supporting patentability. It also protects against competitor challenges that frequently exploit weak or ambiguous data. Low-cost polymorph screening labs, which typically produce noisy PXRD patterns, narrow screening datasets, and limited structural detail, cannot provide the scientific rigor that is required for this complex area of science.
Regulators now emphasize data integrity as strongly as analytical accuracy. They expect raw files, audit trails, metadata, calibration history, and reproducible workflows. Low-cost outsourcing environments often lack these critical components. By contrast, Improved Pharma’s U.S.-based laboratory operates with validated instrumentation, full raw data retention, traceable sample handling, and transparent methods. This ensures that every dataset is fully defensible.
Cost vs Accuracy
The most expensive solid-state problems we see—misassigned forms, incorrect “amorphous” calls, failed reproducibility, and ambiguous PXRD—almost always originate from low-cost work performed with insufficient tools, inadequate oversight, and substandard data interpretation. By combining high-performance in-house PXRD, high-quality SCXRD, access to Argonne’s synchrotron capabilities, orthogonal data and rigorous U.S.-based data governance, and expert interpretation, we provide sponsors with scientific certainty, regulatory confidence, and patent-quality defensibility.
In the world of solid-state chemistry, the certainty associated with high-quality data expert interpretation is worth far more than the illusion of savings offered by low-cost alternatives.
References
Byrn, S. R., G. Zografi and X. S. Chen (2025). Solid-State Materials in Pharmaceutical Chemistry: Properties, Characterization, and Applications, John Wiley & Sons.
Smith, L., Smith, D., Smith, P., Purcell, D., Bogdanowich-Knipp, S., Parent, S., Byrn, S. (2025). “The Use Of Raman Mapping And Hot-Stage Microscopy To Detect Micro-Domains Of Crystallinity In Amorphous Solid Dispersions Of Nilotinib.” AAPS Special Poster Session, San Antonio, TX November, 2025.
Taylor, R. and P. A. Wood (2019). “A million crystal structures: The whole is greater than the sum of its parts.” Chemical reviews 119(16): 9427-9477.
