DOWNLOAD THIS WHITE PAPER AS A PDF BY CLICKING ON THIS LINK
The abuse and misuse of prescription opioid products is a serious and growing public health problem. Opioid products are often manipulated to bypass extended-release (ER) properties. As such, the FDA is encouraging the development of opioid formulations that are more difficult to abuse than previous generations [1]. These abuse-deterrent formulations (ADFs) are designed to be harder to break using common methods of abuse.
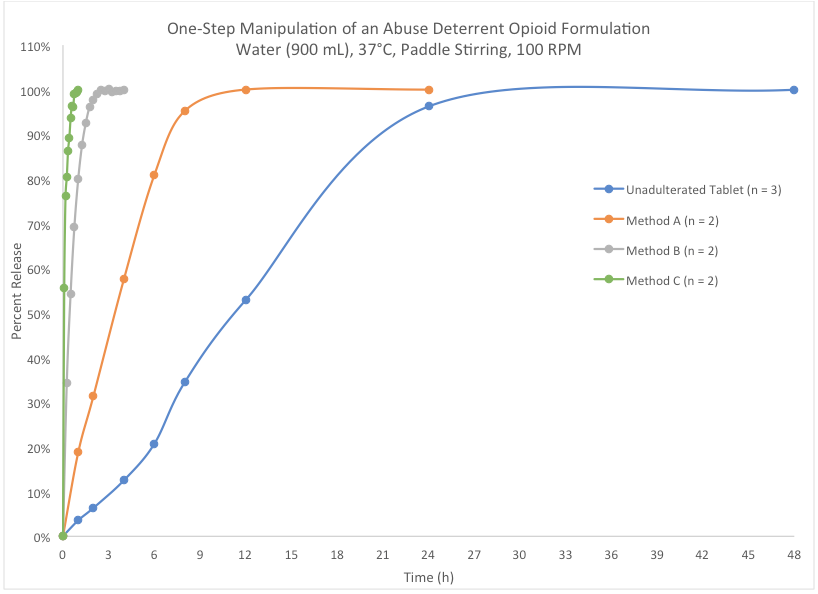
Dissolution testing is one valuable tool for evaluating intact and manipulated formulations. The graph below compares dissolution profiles of three different abuse attempts to that of an unadulterated tablet. For the unadulterated tablet, the opioid is not 100% released until after 24 hours. However, all the abuse attempts significantly increased the release rate, with “Method C” permitting full release in under one hour. Unfortunately, this investigational ADF was not successful at inhibiting abuse of the opioid.
Improved Pharma has significant experience in evaluating ADFs using real-world methodologies. We also offer all the techniques necessary to fully assess a new formulation’s performance and can act as an independent test source for new products. In addition to dissolution testing, we offer tablet hardness, crushablility, syringeability/viscosity, vaporization, particle size analysis, and extractability. We can also assist with formulation design. To learn more, please contact us at info@improvedpharma.com.