The High-Quality Difference: Solid-State Data That Stands Up to FDA and Patent Review
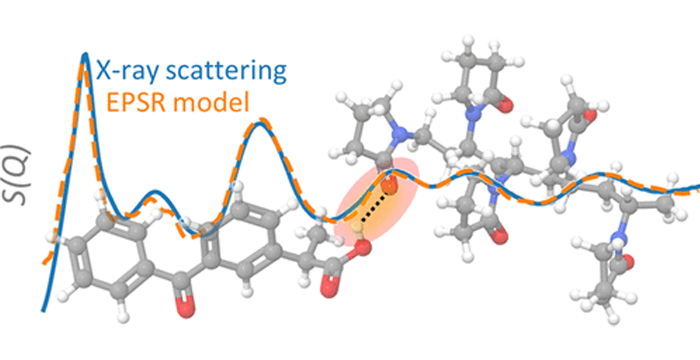
Ed olimpio2026-02-02T15:35:43+00:00Authors Jared Smit, David Engers, Steve Byrn In modern small-molecule development, solid-state characterization is far more than a routine analytical task.(Byrn, Zografi et al. 2025) It defines the manufacturable form of an API, supports Read more