DSC is a powerful method for the analysis of a range of materials. A typical DSC experiment measures the heat changes that occur within the sample when the temperature is changed. For the analysis, a small sample, usually around 5 mg, is placed in a sample holder (a DSC “pan”) and heated at a fixed rate. The instrument then measures thermal transitions, which can be converted to energies (J/g). Thermal events that can occur during this process include melting, loss of solvent or water, the glass transition temperature of amorphous materials, collapse temperature of lyophilized solids, solid state reactions, and phase transitions, including crystallization.
is a powerful method for the analysis of a range of materials. A typical DSC experiment measures the heat changes that occur within the sample when the temperature is changed. For the analysis, a small sample, usually around 5 mg, is placed in a sample holder (a DSC “pan”) and heated at a fixed rate. The instrument then measures thermal transitions, which can be converted to energies (J/g). Thermal events that can occur during this process include melting, loss of solvent or water, the glass transition temperature of amorphous materials, collapse temperature of lyophilized solids, solid state reactions, and phase transitions, including crystallization.
There has been significant interest in using DSC to study lipid nanoparticles in recent years. The remainder of this blog will focus on this area. DSC has also been combined with X-ray powder diffraction (XRPD) analyses to provide additional information on the structure and properties of solid lipid nanoparticles. Importantly, Improved Pharma, LLC is developing a powerful additional method for determining the structure and crystallinity of SLN utilizing a combination of X-ray Pair Distribution Function and solid state NMR analysis.
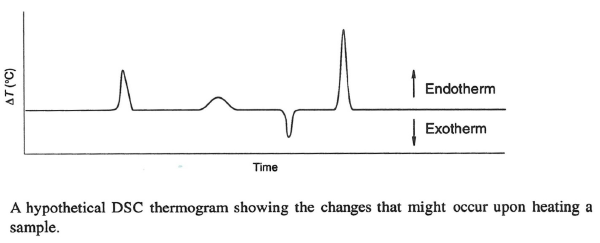
The 1999 book Solid State Chemistry of Drugs, published by SSCI Inc. and authored by S. Byrn, R. Pfeiffer, and J. Stowell shows a typical DSC thermogram containing endothermic and exothermic events. Typically, these peaks are integrated to provide values for the heats of the transformation. This can also provide information on the structure of solid lipid nanoparticles (SLN), such as whether they contain drugs in crystalline or amorphous forms. For SLN, endothermic events are sometimes observed, suggesting melting. Glass transition temperatures are deviations from the baseline and can also be observed for amorphous materials. In rare instances, exotherms for crystallization are observed.
Vijayakumar and coworkers used DSC to study free and quercetin-loaded solid lipid nanoparticles (Vijayakumar et al., 2017). Quercetin itself showed a very high melting above 300 ⁰C, but a physical mixture of quercetin and lipid showed a low melting point of around 60 °C. Similarly, the lipids and the quercetin SLN showed a low melting point of about 55 °C. Based on those observations, the authors suggested that quercetin was amorphous or noncrystalline in the SLN.
Sudhakar, reported a study of ritonavir in stealth liposomes (Sudhakar et al., 2016). Ritonavir itself showed a melting endotherm at about 125 °C, whereas the liposomal product showed a melting point of 66.2 °C. They interpreted this as indicating that the ritonavir was completely amorphous since the drug peak was not observed.
At the University of Texas, Naguib studied SLP formulations of docetaxel prepared with high melting glycerides. (Naguib et al., 2014) They showed that the docetaxel exhibited a melting endotherm of about 167 °C, and a physical mixture of the triglycerides and docetaxel showed a melting point at 143 °C, but docetaxel solid lipid nanoparticles did not show any high-temperature melting point, and only a melting point from the triglycerides around 57 °C. The X-ray diffraction pattern also showed the lack of diffraction from docetaxel in the SLN. Taken together, this shows that in the lipid nanoparticles, docetaxel was in the amorphous or noncrystalline state, whereas in the physical mixture, docetaxel was crystalline. Gordillo-Galeano and coworkers supported this strategy (Gordillo-Galeano & Mora-Huertas 2018). They stated that DSC and X-ray diffraction were the most common ways of analyzing the crystalline nature of solid lipid nanoparticles.
Barman and coworkers developed highly stable nifedipine SLN (Barman et al., 2014). They studied lyophilized SLN in different lyoprotectants using DSC and X-ray diffraction. Some of the lyoprotectants resulted in crystalline nifedipine and others in amorphous nifedipine. In general, the SLN containing crystalline nifedipine, as determined by X-ray analysis, showed a melting peak near the melting point in nifedipine as measured by DSC. This study shows the importance of a combined DSC X-ray study.
Elbink and coworkers reported an interesting study involving the lyophilization of SLN containing the small molecule celecoxib along with with the cryoprotectants trehalose, maltose, and sucrose (Elbrink et al., 2023). In this study, the SLN was prepared by melting celecoxib with glyceryl monostearate and then homogenized with the other lipids. In some cases, crystalline celecoxib was produced in the nanoparticles or appeared when the SLN was subjected to stability stress testing. The formation of crystalline celecoxib as demonstrated by the melt endotherm is considered undesirable since crystalline celecoxib will not release or be bioavailable. Thus, Elbrink used the appearance of the melt endothermic event for crystalline celecoxib in the DSC to indicate instability and product failure. DSC analysis of the celecoxib SLN lyophilized with the cryoprotectants trehalose, maltose and sucrose showed that the trehalose lyophilized SLN did not show the celecoxib melting event even after six months of storage at 4 or 25 ⁰C. In contrast, the celecoxib SLN lyophilized using maltose or sucrose showed a DSC peak corresponding to the melt of crystalline celecoxib after one month of storage, demonstrating instability for these SLN compounds. Thus trehalose was selected as the optimum lyoprotectant.
In conclusion, these studies show that DSC, especially when combined with X-ray diffraction, can be used to study the crystallinity of small molecules formulated in SLN. DSC was also shown to be a good method for analyzing the stability of SLN. One advantage of DSC over X-ray diffraction is the amount of material required since DSC can be easily carried out on 5 mg of material.
