Thermal analysis is an important analytical tool used to study the properties of materials as the temperature is changed. Thermogravimetric analysis (TGA) is a widely used thermal technique in the characterization of crystalline and amorphous pharmaceutical materials. TGA measures changes in sample mass with temperature within a controlled environment. Such measurement provides information concerning the content of volatile materials, such as residual solvent or moisture, in a pharmaceutical material. TGA can also be used to detect dehydration or desolvation for pharmaceutical solvates/hydrates.
TGA can be used to measure changes in the mass of material as a function of time either at a selected temperature (i.e. isothermal mode) or over a particular temperature range using a predetermined heating rate under a controlled atmosphere. This technique is widely used to monitor thermal stability and the loss of volatile components for pharmaceutical materials where a loss of mass occurs because of thermal degradation or desolvation. In desolvation, adsorbed or bound solvents leave the material at elevated temperatures causing a decrease in sample mass, whereas for thermal degradation the loss in mass occurs when volatile degradation products are formed.
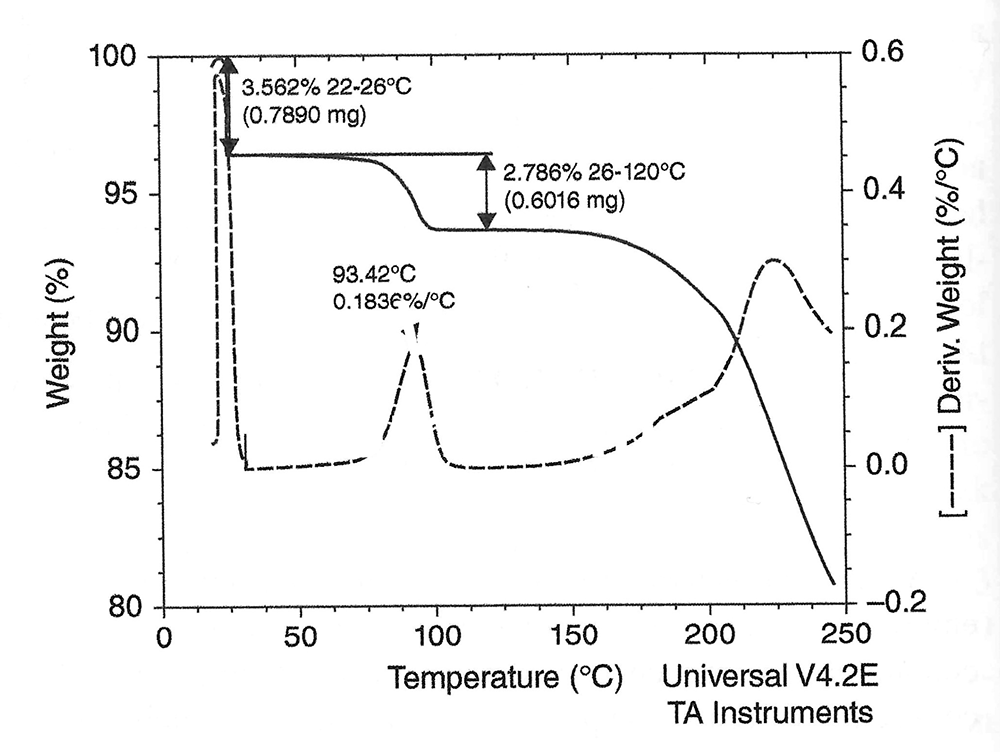
TGA thermal curves are generally displayed with time or temperature as the X axis and weight or weight percent as the Y axis. A typical TGA thermal curve for the dehydration of a sample of SAR474832 hydrate Form 1 is shown in the figure where there are two steps of weight loss, one due to 3.6% dehydration over the range of 22-26 ֯C, and another one with 2.8% weight loss from 60 – 120 ֯C.
DSC and TGA are usually combined together to more thoroughly understand the thermal behavior of a pharmaceutical material. Applications of DSC/TGA for the study of pharmaceuticals include:
- Characterize polymorphs, solvates, hydrates, salts, and cocrystals
- Characterize polymers and other macromolecules
- Characterize amorphous forms and amorphous solid dispersions
- Monitor dehydration and desolvation of crystals
- Optimize the free drying cycle
- Measure chemical purity of organic compounds
- Investigate solid-state reactions
