One of the most critical parts of drug discovery and development is identifying the structure of the drug substance and drug product. For crystalline materials and biologicals it is routine to determine the arrangement of covalent bonds in the drug substance and drug product and the structure of the solid forms of the drug substance. It is also routine to determine the structure of degradation products. It is also critically important to determine what might be called the secondary/tertiary structure of drug substances and drug products since this structure determines important factors like dissolution rate, bioavailability, and stability. Although it is possible to determine the secondary/tertiary structure of solid drug substances if they are crystalline; surprisingly little is known about the secondary/tertiary structure of noncrystalline and amorphous drug substances and drug products. Are they ordered? For multicomponent systems, are they homogeneous or heterogeneous? Do they contain domains?
Pair distribution function (PDF) has been established as a powerful method to determine the secondary/tertiary structure of noncrystalline inorganic materials and mixtures including the molten radioactive amorphous mixture present at the site of the Chernobyl nuclear disaster[1]. Improved Pharma and Purdue University researchers have pioneered the utilization of PDF to determine the secondary/tertiary structure of amorphous and noncrystalline pharmaceutical materials. This structure determination service is now available through Improved Pharma, LLC a research and Information Company in West Lafayette, IN.
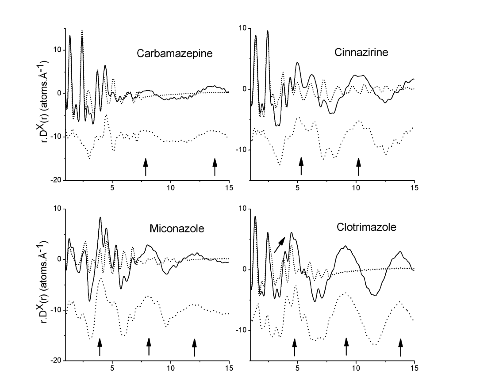 In a 2013 paper using highly accurate PDF measurement we showed that different amorphous and liquid crystalline drug substances had greatly different PDFs and difference-PDFs. These patterns are shown in the insert[2]. These difference clearly show the sensitivity of PDF to structure. These differences are also consistent with the wealth of knowledge on inorganic materials and outlined in Simon Billinge’s book entitled “Underneath the Bragg Peaks”. (Professor Billinge and Improved Pharma have established a collaboration agreement.) At the AAPS meeting in 2015, Improved Pharma, Janssen Pharmaceuticals (Part of J&J), Panalytical, and Argonne National Laboratories, presented a poster showing how the PDF could be used to verify that the structure of different dispersions (drug plus polymer) was similar regardless of the spray drying conditions[3]. This report illustrates the power of PDF for determining sameness and will be discussed in detail in a subsequent application note. Then, in a recent paper in 2018, the same team from Purdue showed that PDF of HPMCP dispersions were different from the PDF for HPMC dispersions and that this difference was related to salt formation and drug loading[4]. Further, the dispersion with the greatest amount of salt formation was the most stable. This study will also be discussed in more detail in a subsequent application note.
In a 2013 paper using highly accurate PDF measurement we showed that different amorphous and liquid crystalline drug substances had greatly different PDFs and difference-PDFs. These patterns are shown in the insert[2]. These difference clearly show the sensitivity of PDF to structure. These differences are also consistent with the wealth of knowledge on inorganic materials and outlined in Simon Billinge’s book entitled “Underneath the Bragg Peaks”. (Professor Billinge and Improved Pharma have established a collaboration agreement.) At the AAPS meeting in 2015, Improved Pharma, Janssen Pharmaceuticals (Part of J&J), Panalytical, and Argonne National Laboratories, presented a poster showing how the PDF could be used to verify that the structure of different dispersions (drug plus polymer) was similar regardless of the spray drying conditions[3]. This report illustrates the power of PDF for determining sameness and will be discussed in detail in a subsequent application note. Then, in a recent paper in 2018, the same team from Purdue showed that PDF of HPMCP dispersions were different from the PDF for HPMC dispersions and that this difference was related to salt formation and drug loading[4]. Further, the dispersion with the greatest amount of salt formation was the most stable. This study will also be discussed in more detail in a subsequent application note.
In conclusion, it is clear that PDF is a powerful method for structural analysis of amorphous and noncrystalline materials. This method can be used to provide characterization information on previously uncharacterized pharmaceutical materials and can lead to a better understanding of sameness and stability information. Finally, this method provides a way to describe these materials in patents for intellectual property purposes.
References:
[1] Skinner, L. B.; Benmore, C. J.; Weber, J. K. R.; Williamson, M. A.; Tamalonis, A.; Hebden, A.; Wiencek, T.; Alderman, O. L. G.; Guthrie, M.; Leibowitz, L.; et al, Science (Washington, DC, United States) (2014), 346(6212), 984-987.
[2] Benmore, Chris J.; Weber, J. K. R.; Tailor, Amit N.; Cherry, Brian R.; Yarger, Jeffery L.; Mou, Qiushi; Weber, Warner; Neuefeind, Joerg; Byrn, Stephen R., Structural characterization and aging of glassy pharmaceuticals made using acoustic levitation, Journal of Pharmaceutical Sciences (2013), 102(4), 1290-1300.
[3] Hector Novoa de Armas1, Marcus Brewster1, Detlef Beckers2, Milen Gateshki2, Chris Benmore3, Stephen Byrn4 (1 Pharmaceutical and Material Sciences, Johnson & Johnson Pharmaceutical Research & Development, a division of Janssen Pharmaceutica NV, Turnhoutseweg 30, 2340 Beerse, Belgium, 2 PANalytical B.V. Lelyweg 1 (7602 EA) PO Box 13, 7600 AA, Almelo, The Netherlands, 3 Argonne National Laboratory, 9700 S. Cass Ave, Argonne, IL 60439, USA,4 Department of Industrial and Physical Pharmacy, Purdue University, 575 Stadium Mall Drive, West Lafayette, IN 47906, USA), Stability and comparability of an amorphous drug prepared by different spray drying processes: atomic Pair-wise distribution functions (PDF) using conventional X-ray diffraction versus high energy synchrotron radiation, AAPS National Meeting, San Diego, CA, 2015.
[4] de Araujo, G. L. B.; Benmore, C. J.; Byrn, S. R., Local Structure of Ion Pair Interaction in Lapatinib Amorphous Dispersions characterized by Synchrotron X-Ray diffraction and Pair Distribution Function Analysis. Sci. Rep. 2017, 7, 46367.