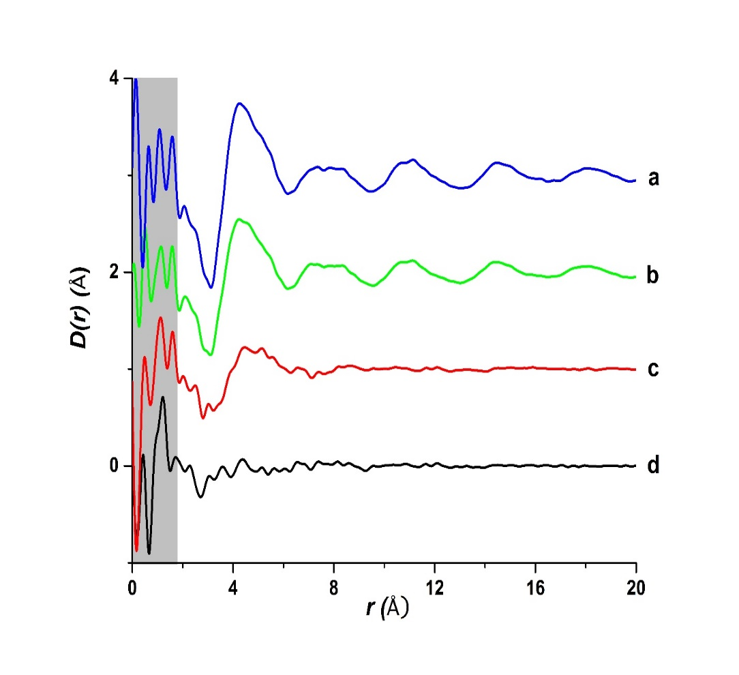
Structure of amorphous formulations at the angstrom level using PDF
Pair distribution function (PDF) analysis of amorphous materials is a well-established technique having been used for many years in the physics and materials science community. Our research team at Purdue began using this method in the early 2000s for analysis of the structure of pharmaceutical liquid crystals. Then in the last few years, our team has used this method for amorphous pharmaceutical materials and pharmaceutical formulations. Recently, our team published a major paper in Nature Scientific Reports (de Araujo, G. L. B., Benmore, C.J., Byrn, S.R., Local Structure of Ion Pair Interaction in Lapatinib Amorphous Dispersions characterized by Synchrotron X-Ray diffraction and Pair Distribution Function Analysis. Nature Sci. Rep. 7, 46367; doi: 10.1038/srep46367 (2017). This paper showed that for lapatinib (a weak base containing a secondary amine) only an amorphous dispersion with the salt forming polymer HPMCP gave a dispersion devoid of the lapatinib-lapatinib interactions present in the pure amorphous drug and in HPMC dispersions. Furthermore, stability studies showed that pure lapatinib and the HPMC dispersions crystallized but the amorphous dispersion devoid of molecule-molecule interactions was stable to crystallization. This study also showed that pair distribution function analysis can determine the structure of amorphous dispersions and formulations down to the angstrom level. Heretofore, the highest resolution technique was solid-state NMR relaxation times which was able to determine domain sizes an order of magnitude larger (10 to 300-angstrom level).
Improved Pharma provides services that can determine the structure of your dispersion using this same method. Our approach allows selection of the most stable formulation without long-term stability studies. Improved Pharma can also carry out solid-state NMR studies or other analytical testing on dispersions if requested.