Solid State Chemistry of Long-Acting Injectable Suspensions
Steve Byrn
September 1, 2025
The Enduring Impact of Long-Acting Injectable Suspensions in Medicine
Long-acting injectable (LAI) suspensions play a crucial role in modern drug delivery, offering ingenious product designs for diverse medical conditions. Their utility spans critical therapeutic areas for treating conditions such as schizophrenia, bacterial infections, and viral infections, significantly enhancing patient health and treatment adherence.
A Century of Progress: The Evolution of Injectable Suspensions
The journey of injectable solutions is a testament to scientific innovation, as illustrated in Figure 1. The first non-crystalline injectable suspension was introduced in 1912 for syphilis treatment. The 1920s saw the introduction of crystalline bismuth salts also developed for syphilis treatment.[1] A major leap occurred in the 1930s with the development of protamine-zinc-insulin suspections for diabetes management.[2] This progress continued with NPH insulin suspensions in 1946 and crystalline ultralente insulin in 1952.[3, 4] The 1960s marked the advent of decanoate antipsychotics and other poorly soluble salt forms as LAIs.[5, 6] Procaine penicillin , a long-acting suspension for infectious diseases, followed in 1968. Later milestones include Lupron Depot, a treatment for cancer, in 1989 and paliperidone pamoate in 2009.[7] More recently, significant advancements in HIV treatment have been introduced with Sunlenca, a once-every-six-month injectable crystalline suspension, and Apretude, administered once every two months.
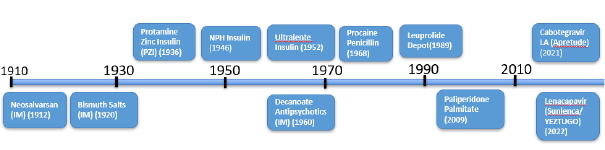
Figure 1. Timeline for injectable suspensions
While many products in Figure 1 are non-crystalline (e.g., NPH insulin, decanoate antipsychotics, Lupron depot, and paliperidone palmitate), their sustained release is likely achieved through vehicle-based kinetics, rather than crystallization at the injection site, for which evidence remains sparse.
The Promise of Crystalline Long Acting Injectable Suspensions
Crystalline injectable suspensions are particularly compelling due to their precise control of drug release, which can be finely tuned by adjusting particle size and vehicle composition. Figure 2 highlights key developments in this area.
The earliest slow-release crystalline injectable product was a bismuth salt in 1920.[1] In 1936, protamine-zinc insulin offered a slower release compared to insulin solutions.[2] Sixteen years later, ultralente insulin emerged as another long-acting crystalline product.[3] Procaine penicillin introduced in 1968, incorporated procaine to alleviate injection pain while leveraging a crystalline antibiotic for long-lasting release.[6] After a considerable gap, paliperidone pamoate was introduced in 2009, followed by aripiprazole monohydrate in 2013.[7] Recent breakthroughs include the introduction of crystalline cabotegravir (administered every 2 months) in 2021 and crystalline lenacapavir (administered every 6 months) in 2022.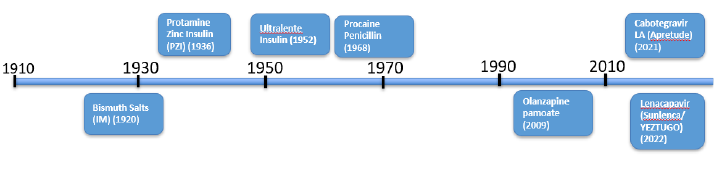
Figure 2. Timeline of crystalline long acting injectable suspension formulations.
Remenar’s review of the transition from daily oral dosing to LAIs underscores their importance, particularly for for schizophrenia, where they significantly reduce relapses by maintaining stable drug levels .[7] Early LAIs were often oily or lipid-based solutions and prodrugs. The 1960s saw the introduction of noncystalline long-acting antipsychotic injectables. Around 2009, aqueous crystalline suspensions began to emerge. Remenar highlighted aripiprazole monohydrate and olanzapine pamoate as pivotal crystalline formulations.
Aripiprazole monohydrate exemplifies the careful study of crystallinity in LAIs. This suspension demonstrates remarkable stability, maintaining its crystal structure after 3 months at 40°C/75% RH as shown in Figure 3.[8]
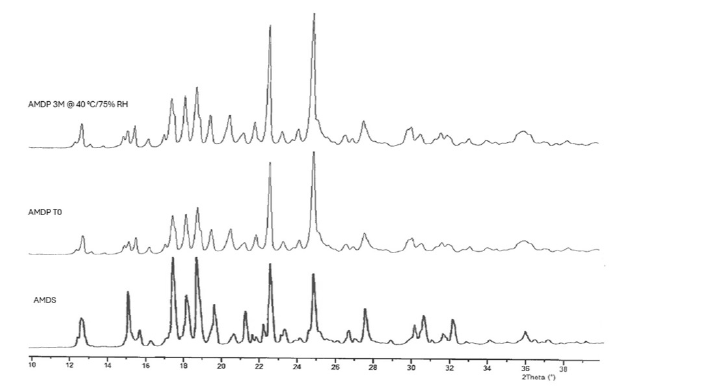
Figure 3. Exemplary X-ray powder diffractograms of AM in powder form, before formulation process (AMDS), AM (Aripiprazole monohydrate in drug product after lyophilization, before stability study (AMDP TO), and AM in drug product after 3 months of stability study at accelerated conditions.[8]
Similarly, brexpiprazole, another intramuscular suspension, contains crystalline drug, confirmed via X-ray powder diffraction, and its crystal structure remains intact after injection.
Key Factors Influencing the Release and Performance of Crystalline LAIs
The performance and release profile of long-acting crystalline injectable suspensions are influenced by a complex interplay of factors. While depot formation at the injection site is sometimes observed with crystalline suspensions, it is not clear whether this is always the case. Cabotegravir, a crystalline suspension, is known to form a depot, leading to slow drug release due to poor solubility. A combination cabotegravir/ rilpivirine, both crystalline drugs, also exhibit prolonged action through depot formation.
Pharmacokinetic analysis reveals particle size and solid form significantly influence the release profiles of a crystalline injectable formulation. The route of administration also plays a role, with many products being administered subcutaneously while others, like procaine penicillin, are administered intramuscularly.
Particle size is paramount. For example, Ultralente insulin is a microcrystalline zinc insulin suspension, features particles smaller than 25 µm. X-ray diffraction confirms it exists in the crystalline space group R3, indicative of an insulin hexamer with no evidence of amorphous insulin. Its production involves precise pH adjustment and precipitation, and the original composition and processes protected by patents.
The formulation and vehicle are equally crucial. Methylprednisolone acetate (40 or 80 mg), used for various allergic, dermatological, GI, and neoplastic diseases, is formulated for sustained release with polyethylene glycol (PEG) and a myristyl chloride derivative. Kenalog (triamcinolone), a topical crystalline product, achieves sustained release through a formulation that includes sodium chloride, benzyl alcohol, carboxymethylcellulose, and polyols. The release of procaine penicillin, a crystalline long-acting intramuscular injection, is significantly affected by its vehicle, which can be either aqueous or oily. Other factors, such as viscosity, injectability, surfactants, wetting agents, and polymer coatings, also influence release mechanism. Maintaining drug stability in the vehicle is also critical.
Buckwalter’s research on procaine penicillin identified peanut or sesame oil gels with 2% aluminum monostearate as optimal. He noted a 5 µm particle size superior clinical results in studies involving over 1,000 patients. He also authored US Patent 2,507,193 on injectable salt suspensions. In contrast, a risperidone formulation, confirmed as amorphous by XRPD, still provides sustained release, likely due to its vehicle.
Holistic Development: From Concept to Product Finalization
In summary, Figure 4 describes these and other factors to consider in developing LAIs. Early development hinges on five key variables:
- Vehicle: Could be polymeric, a viscous oil, or a crystalline salt.
- Salt form and polymorphism: For example, aripiprazole has both hydrated and salt forms.
- Particle size: Large particles yield slow release; smaller particles release more rapidly.
- Intrinsic solubility: Determines the volume needed to dissolve the drug.
These properties influence release mechanisms and profiles, measured via in vitro dissolution or in vivo models. Downstream development (Product Finalization) factors include:
- Sterilization method
- Packaging and stability
- Administration method (needle size, volume)
- Local tolerability at the depot site
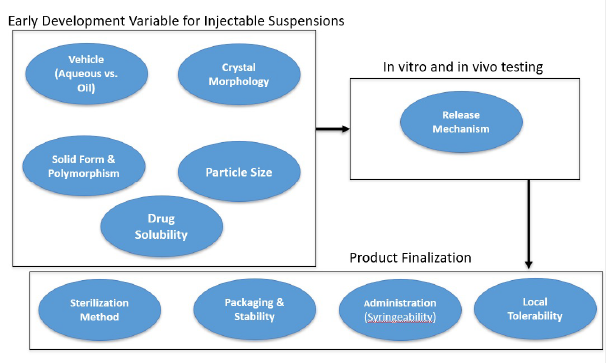
Figure 4. Factors affecting early development, in vitro and in vivo testing and product finalization of a long acting crystalline injectable suspension.
Sterilization of LAIs presents unique challenges. Terminal methods like autoclaving can degrade the drug or alter the crystal form. Sterile filtration is rarely feasible due to particle size, unless submicron particles are used, often requiring alternative methods like irradiation or gas exposure. Polymorphism and solid-state stability are also crucial, as interconversion may occur over time. Formulating with the most stable polymorph is generally desirable; for instance, hydrates tend to be more stable than anhydrous forms, explaining the use of aripiprazole monohydrate. Release kinetics, influenced by the formulation, polymorph control, or prodrug conversion, must be rigorously evaluated using standard in vitro and in vivo techniques. Additionally, prodrugs can offer additional flexibility and optimized release profile.
Conclusion
The successful development of long-acting injectable suspensions requires a comprehensive understanding of drug properties and the interplay of the factors discussed above. At Improved Pharma, we possess the experience and tools necessary to navigate these complexities and guide the development of the advance drug delivery systems. We invite you to contact us to explore potential collaborations.
References
- Ioannou P: The use of bismuth nitrate pentahydrate, Bi (NO3) 3ċ5H2O, and bismuth subnitrate monohydrate, BiO (NO3) ċH2O, for the preparation of tris (arylthio) bismuthines. Main Group Chemistry 2011, 10:255-264.
- Lawrence R, Archer N: Some experiments with protamine insulinate. British Medical Journal 1936, 1:747.
- Hallas-Møller K, Petersen K, Schlichtkrull J: Crystalline and amorphous insulin-zinc compounds with prolonged action. Science 1952, 116:394-398.
- Hallas-Mø K: The lente insulins. Diabetes 1956, 5:7-14.
- Buckwalter F, Dickison H: The effect of vehicle and particle size on the absorption, by the intramuscular route, of procaine penicillin G suspensions. Journal of the American Pharmaceutical Association (Scientific ed) 1958, 47:661-666.
- Buckwalter FH: Penicillin Product. US Patent 2,507,133.
- Remenar JF: Making the leap from daily oral dosing to long-acting injectables: lessons from the antipsychotics. Molecular pharmaceutics 2014, 11:1739-1749.
- Pietrzak T, Szendzielorz Z, Borychowska J, Ratajczak T, Kubisiak M: The Impact of a Manufacturing Process on the Stability of Microcrystalline Long-Acting Injections: A Case Study on Aripiprazole Monohydrate. Pharmaceutics 2025, 17:735.
