In our last blog, we explored the importance of control strategies during early pharmaceutical development. However, two additional elements critical to early-stage Investigational New Drug (IND) applications deserve special attention: process validation and method validation. At Improved Pharma, we integrate these three components – control strategy, process validation, and method validation – to ensure consistent quality from the earliest stages.
Early Validation: Establishing Confidence
Early validation in IND-enabling development is not about confirming full-scale commercial readiness but a proactive and strategic approach to establish confidence in the manufacturing product, process and test methods. As defined in regulatory guidelines and comprehensive texts like “Pharmaceutical Process Validation” (Nash & Wachter, 2003), early validation involves controlled small-scale studies to gather preliminary evidence and demonstrate proof-of-concept for critical aspects before significant resources are invested in later-stage development and formal validation.
At Improved Pharma, our initial focus is on small-scale API processing, crystallization, and amorphization, ensuring the consistent formation and stability of the most desirable solid form of the drug substance. We rigorously employ techniques such as XRPD and thermal analysis to verify solid form consistency and identify the most stable structure, laying a well-understood foundation for pharmaceutical development.
Drug Product Development: Early Integration and Testing
Transitioning from drug substance to drug product, our team conducts small-scale manufacturing batches to assess process feasibility and establish reproducibility. These batches undergo rigorous dissolution and release profile assessments to verify performance consistency.
Occasionally, co-processing – combining drug substance and drug product manufacturing steps – helps expedite early-phase development. Even then, comprehensive analytical testing remains essential.
We rely extensively on XRPD, DSC, high-performance liquid chromatography (HPLC and UPLC), and LC-MS/MS for accurate identification, impurity profiling, and early detection of degradation products.
De-Risking Through Early Validation
Early process validation is as much about problem-solving as it is about validation itself. We proactively assess risks associated with polymorphic interconversion, degradation, and inadvertent amorphization/crystallization during manufacturing. Employing design of experiments (DoE) and tools such as failure mode and effects analysis (FMEA), we methodically and proactively explore risks in the product, process and test methods, establishing robust control parameters and defining provisional critical quality attributes (CQAs) and critical process parameters (CPPs).
Method Validation: Fit-for-Purpose in Early Phases
In parallel, we perform initial method evaluations specifically tailored for early development phases. While regulatory expectations, such as those outlined in ICH Q6 and Q8 guidelines, do not mandate full ICH Q2(R2) validation at this stage, they emphasize fit-for-purpose method qualification. Further, early validation applies sound analytical development principles guided by ICH Q14, as an overarching science and risk-based approach for how test methods should be developed. At Improved Pharma, our approach verifies analytical methods for specificity, accuracy, precision, and sensitivity, aligning closely with the intended clinical dosage ranges and product requirements.
Typical methods qualified at this stage include:
- XRPD and DSC for solid-state form characterization
- HPLC/UPLC for potency and impurity quantification
- LC-MS/MS for precise impurity and degradation analysis
An Integrated Approach for Early Development Success
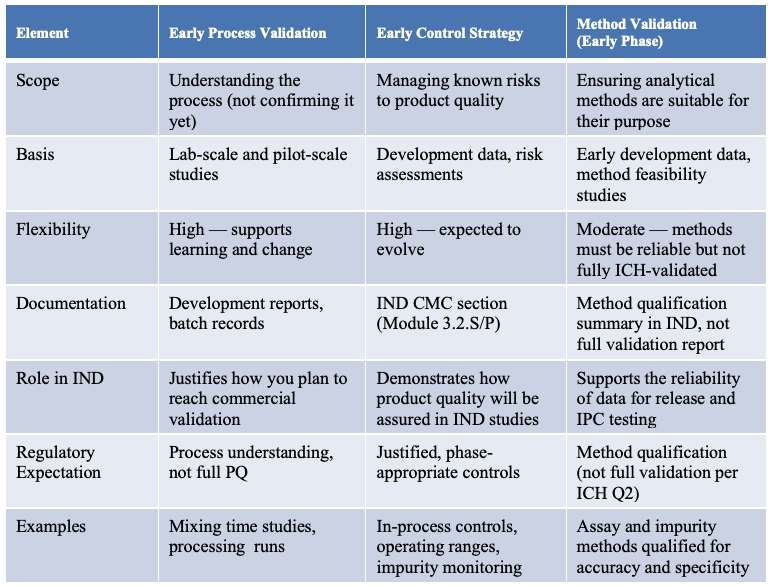
The seamless integration of process validation, method validation, and control strategies establishes a comprehensive framework supporting early IND submissions. A clearly structured approach (Table 1) illustrates how these three critical elements interrelate – covering their scope, documentation, regulatory expectations, and practical examples.
This integrated approach facilitates intellectual property development on important and critical aspects of the process.
Facilitating Tech Transfer and Regulatory Confidence
By investing strategically in these early validation efforts, we lay a robust foundation for successful technology transfer and efficient scale-up in GMP environments. The resulting process consistency, analytical test method reliability, and defined control strategies ensure reproducibility, regulatory compliance, and quality assurance, significantly de-risking clinical manufacturing and enabling smoother regulatory interactions. The data generated during early validation provides crucial information and justification for the proposed manufacturing process and analytical methods in the IND application.
In summary, early-stage validation activities at Improved Pharma aren’t merely about achieving immediate milestones; they set a durable foundation that sets the trajectory for long-term product success, enabling confident and efficient progression toward commercialization.
Table 1. Integrated approach to Early Validation