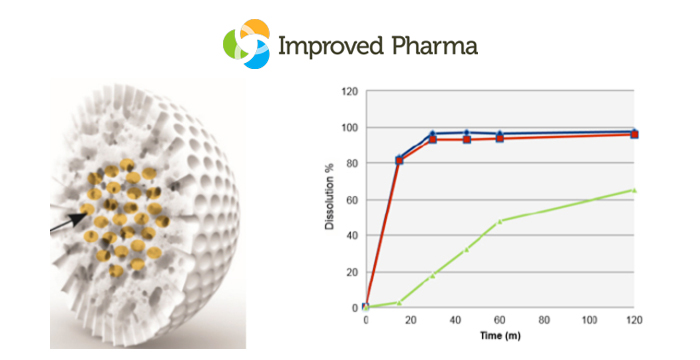
At AAPS PharmSci 360 it was clear that new excipients could be utilized to innovatively solve a range of amorphous dispersion/formulation problems. These new excipients provide significant strategy advances over conventional approaches and although our Improved Pharma scientists utilized a number of novel approaches to prepare amorphous dispersions while at SSCI, these new excipients provide even more innovative strategies. Figure 1 shows the new WR Grace Syloid® and the enhanced dissolution profile this excipient can afford.
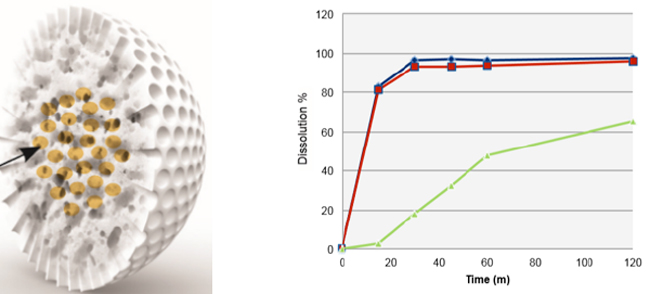
Figure 1. Left panel: Impregnated Syloid. Right panel: Dissolution profile
In a very interesting paper Dave and co-workers showed that combining Syloid® with a polymer provided even greater dissolution rate enhancement. Kinnari and coworkers also showed that Syloid® was very effective in producing a composition of itraconzazole with signifcantly enhanced dissolution and faster release. Overall, this excipient along with Improved Pharma’s analytical methods provides a new/improved strategy to further enhance dissolution and stabilize amorphous materials. Additionally, Improved Pharma’s PDF Synchrotron methods provide a way to determine the structure of these new materials and enhance the ability to describe these compositions both in regulatory and patent applications.
References:
Figures provided from WR Grace brochure entitled “Syloid XDP Silica Pharmaceutical excipient (https://grace.com/personal-care/en-US/Documents/Syloid%20XDP%20Application%20Note.pdf).
Patel and Dave, AAPS PharmSciTech, Vol. 14, No. 2, June 2013 (# 2013) DOI: 10.1208/s12249-013-9947-z
Kinnari, P., et. al, Int. J. Pharm., 414, 148-156 (2011).