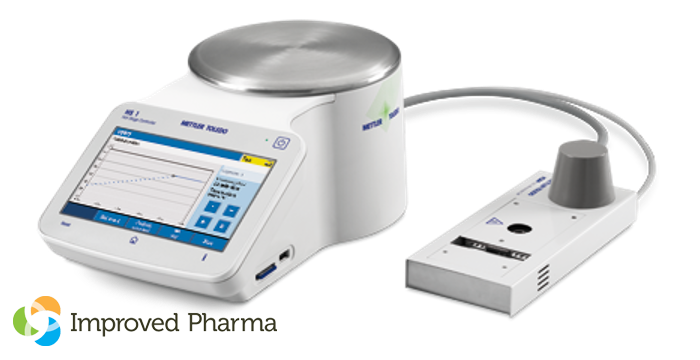
 WEST LAFAYETTE, Ind., Feb. 12, 2024 /PRNewswire-PRWeb/ — Improved Pharma is pleased to announce expanded capabilities in collaboration with Chemical Microscopy for evaluating the thermal properties of micro-quantities of materials. The newly-added Mettler-Toledo HS84/HS1 DSC controller micro-DSC system is the only true DSC hot stage on the market, and will allow visual observation of thermal events when only very small amounts of material are available for use during early stages of development.
WEST LAFAYETTE, Ind., Feb. 12, 2024 /PRNewswire-PRWeb/ — Improved Pharma is pleased to announce expanded capabilities in collaboration with Chemical Microscopy for evaluating the thermal properties of micro-quantities of materials. The newly-added Mettler-Toledo HS84/HS1 DSC controller micro-DSC system is the only true DSC hot stage on the market, and will allow visual observation of thermal events when only very small amounts of material are available for use during early stages of development.
Both qualitative and quantitative information can be obtained by monitoring either chemical or physical changes in the sample which requires only ~200-500 micrograms for analysis. The micro-DSC has an operating temperature range of 25 °C to ~350 °C and can easily be used up to 325 °C with an objective on one of our in-house traditional, IR or Raman microscopes.
“We are very excited to bring the micro-DSC capability into our labs,” states Dr. Stephen Byrn, CSO of Improved Pharma. “Having the appropriate expertise to correctly work with and accurately analyze very small quantities of materials is crucial in both early stages when sample size is limited, and late stages of pharmaceutical development, when a new form may unexpectedly pop-up.” Dr. Pamela Smith, COO of Improved Pharma adds, “We are fortunate to be able to collaborate with very talented internal scientists, who have incredible knowledge and skill sets required to understand and identify potential issues during lead candidate development.”
The addition of the micro-DSC hot stage complements Improved Pharma’s current thermal analytical and microscopic capabilities. This unique micro-technique will support the growing list of Improved Pharma’s solid-state form capabilities, including microscopy-based polymorph screens and problem solving capabilities, and will also be used during fit-for-purpose polymorph, salt, and co-crystal screens, monitoring stability, and litigation support.
About Improved Pharma
Improved Pharma is a research and information company dedicated to improving pharmaceutical methods, formulations, and processes. Services include solid-state form studies, formulation design, synchrotron techniques, analytical testing, and expert consulting for the development and defense of intellectual property matters. The company was founded in 2006 by Stephen and Sarah Byrn, who also founded SSCI.
For more information about the analytical technique discussed in this press release or about Improved Pharma’s services, please contact us at 1-765-463-9951 or info@improvedpharma.com.
