Accelerated development is a complex and difficult task. S-fIND (Synchrotron-based fast to IND) is a strategy to speed development of the most difficult cases using powerful X-ray methods only available at a Synchrotron X-ray source. S-fIND integrates powerful methods with practical pharmaceutical development approaches and excellent people in a package that accelerates development.
Accelerated development services and approaches have been in operation for many years at top pharmaceutical companies. Eli Lilly and Company developed a spin-off company called Chorus R&D that showed that accelerated development approaches using subcontractors could save millions of dollars in development costs. Chorus described a strategy to develop drugs through proof of concept in about 30 months for $3 million dollars. Another reason top pharmaceutical companies utilize accelerated development is to reduce the time to reach the important Go-No Go decision [point enabled by filing an IND]. At about the same time SSCI, Inc. in West Lafayette, Indiana developed a fast to IND service named INDiGO (to my IND I go). INDIGO is the brainchild of Professor Stephen R. Byrn of Purdue University, who also founded SSCI. INDiGO is currently an Evotec product and one report indicates Evotec has over 20 customers for INDiGO mostly in Europe.
Now Improved Pharma, another West Lafayette, Indiana firm founded by Professor Byrn and located in the Purdue Research Park, is offering a powerful new fast to IND service based on the utilization of Synchrotron X-rays (S-fIND). Improved Pharma’s S-fIND service is superior to existing accelerated development services because of its utilization of advanced analytical, formulation, and microscopic-based CMC capabilities. The S-fIND platform utilizes Synchrotron X-ray methods to solve complicated development problems including:
- Complex polymorph analysis and control issues
- Polymorphic and solid-state transformations during formulation
- Polymorphic and solid-state transformations during stability
- Poor solubility
- Intellectual property related issues
Improved Pharma has already utilized the S-fIND platform to solve problems such as: (1) appearance of a new undetected polymorph late in development; (2) Detection of small amounts of amorphous material in otherwise crystalline API lots; (3) Existence of domains in spray dried amorphous dispersions that were intended to contain molecular mixtures; and (4) Analysis of the structure of spray dried dispersions prepared using different conditions, different drug loadings, and different polymers.
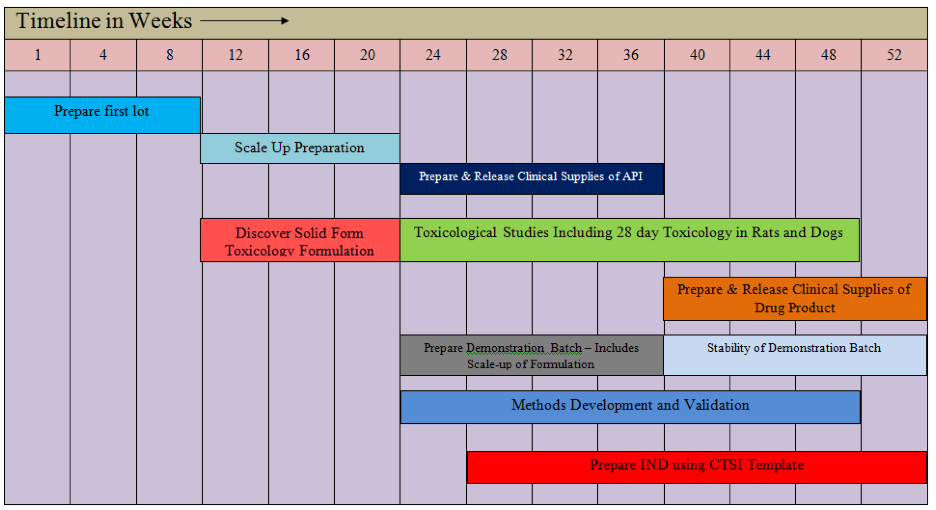
Additional advantages of the S-fIND platform include the much higher resolution and sensitivity of Synchrotron-based X-ray analytical methods allowing detection of new polymorphs obscured by other forms in a complex mixture, and also the identification of the polymorphs(s) present in a formulation before and after stability. The diagram below (Figures 2) illustrates the timing of the S-fIND program.
Solving solid state problems is the lynchpin in fast development. Merck typically uses the solid form produced in early experiments for the IND provided it is bioavailable. Finding the right solid forms for these experiments including animal experiments can greatly accelerate development and avoid costly bridging studies.
Figure 1 outlines the overall strategy for S-fIND. As shown in Figure 1, synchrotron X-rays are a key element allowing highly sensitive detection of new polymorphs and analysis of the polymorph/form in the formulation. This information is critical to preparing clinical supplies for an IND. Knowing the form and finding a stable form is the lynchpin to accelerate development.
Formulation design is the second key element in accelerating development. Armed with the powerful Synchrotron methods, formulation design for IND clinical supplies can proceed rapidly.
Problem solving is perhaps Improved Pharma’s strongest capability. Improved Pharma, because of its vast experience in the field, is capable of solving almost any development problem. Improved Pharma, because of its experience and contacts has developed a range of strategies for interdisciplinary integration especially as it relates to solid state chemistry integrated with API synthesis, formulation development and manufacturing.
Finally, Improved Pharma has excellent people with over 70 years of experience in accelerated development and extensive experience with project management. Improved Pharma utilizes only the top subcontractors for GMP manufacturing and preclinical animal studies.
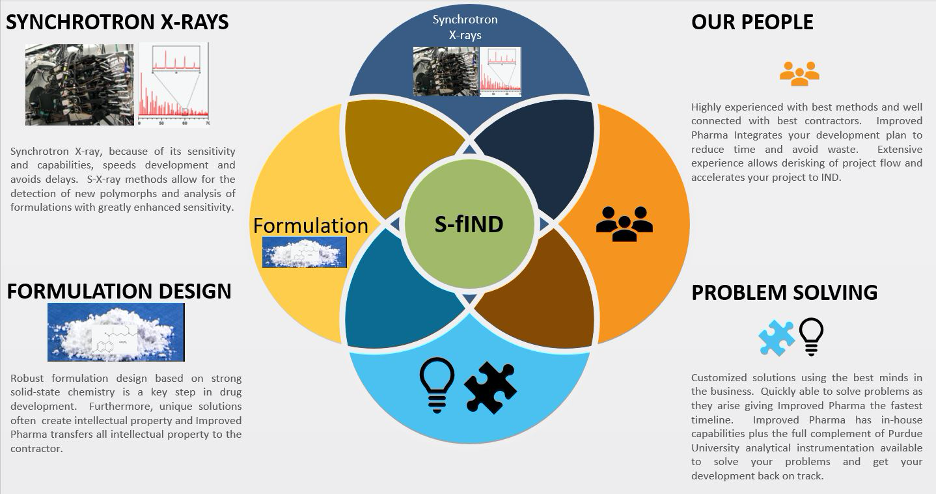
Figure 1. Diagram showing the main features of S-fIND (www.presentationgo.com)
Figure 2 outlines in more detail how Improved Pharma accelerates development following the fastest timeline in the industry. This timeline is referred to as a skyway to success. First, a custom IND development plan is established using Improved Pharma’s expertise along with extensive client input.
From its laboratory in the Purdue Research Park, Improved Pharma internally does the formulation, dissolution, HPLC analysis, and manufacture of the demonstration batch. Synchrotron X-ray data are collected by Improved Pharma at Argonne National Laboratory, a 2-hour drive from West Lafayette. They also develop formulation methods and prepare formulations for toxicology studies. Improved Pharma contracts to outstanding contractors located within 500 miles of the Purdue Research Park for GMP API manufacturing, GMP drug product manufacturing, packaging of clinical supplies, and preclinical animal studies. In this diagram the top three bars illustrate the API manufacturing. Once the first lot is available after 12 weeks, Improved Pharma, working with quantities as small as 50 mg develops preliminary formulations and does analysis using Synchrotron X-ray diffraction. Improved Pharma has industrial access to three Synchrotrons units, one at Argonne National Laboratory, one at Brookhaven National Laboratory (easily accessible by a direct flight to New York City) and one at the Swiss Light Source operated by our collaborator Excelsus Structural Solutions. Once the formulation is developed, it is provided to the toxicology lab for preclinical studies and scale-up and methods development work takes place. By this time (at the 20th week) larger quantities of API are available allowing formulation scale up, manufacture of a demonstration batch, and methods implementation. At the 36th week, GMP manufacturing can take place and the required stability studies initiated. Meanwhile the stability of the demonstration batch will be available as well as information from stress testing and excipient compatibility testing. This information along with accelerated stability studies on the GMP lot provides enough information to package and ship clinical supplies once the IND is approved. Additionally, from the 36th week on, Improved Pharma’s regulatory consultants are preparing the IND, contacting the FDA and completing the investigators brochure. Once the IND is filed, Improved Pharma provides complete knowledge transfer to enable manufacture of Phase 2 clinical supplies as needed.
In addition to the general strategies outlined above Improved Pharma has specific experience with a wide range of technologies for solving problems. These include:
- Increasing bioavailability by discovering the best enabled formulation including amorphous formulations, solid lipid nanoparticles, spray dried formulations with drug-polymer complexes
- Finding improved salts for development
- Controlled release matrix formulations
- Sorting out complex polymorph problems and developing the best method for controlling the manufacturing to produce the desired polymorph
In conclusion, S-fIND offers accelerated development coupled with the most powerful analytical methods and tailored to each customer’s needs. Please contact Improved Pharma to arrange for a review of your project and obtain a ballpark quote.
