DEA registration allows Improved Pharma to support pharmaceutical development for all types of controlled substances.
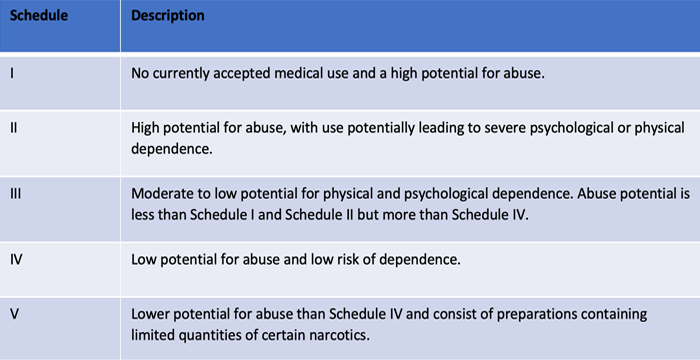 Improved Pharma is pleased to announce their DEA registration for schedules I – V. The new controlled substance license enhances the wide variety of drug substances and drug products that can now be evaluated during fit-for-purpose polymorph, salt, and co-crystal screens, formulation support and design, abuse deterrent testing, analytical testing, microscopy, and litigation support.
Improved Pharma is pleased to announce their DEA registration for schedules I – V. The new controlled substance license enhances the wide variety of drug substances and drug products that can now be evaluated during fit-for-purpose polymorph, salt, and co-crystal screens, formulation support and design, abuse deterrent testing, analytical testing, microscopy, and litigation support.
“We are pleased to be able to contribute to the pharmaceutical development research being conducted on these very important molecules. With this registration, we can better support all our clients”, remarked COO Pam Smith.
About Improved Pharma
Improved Pharma is a research, consulting, and information company dedicated to improving pharmaceutical methods, formulations, and processes. Services include solid-state form studies, formulation design, synchrotron techniques, analytical testing, and expert consulting for the development and defense of intellectual property matters. The company was founded in 2006 by Stephen and Sarah Byrn, who also founded SSCI.
