- Smith1, C.J. Benmore2, J.K.R. Weber2,3 and S.R. Byrn1.
1) Improved Pharma, West Lafayette, IN 47906, USA
2) X-Ray Science Division, Advanced Photon Source, Argonne National Laboratory, Argonne, IL 60439, USA
3) Materials Development, Inc., Arlington Heights, IL 60004, USA
The kinetics and disorder of hydration (or dehydration) of pharmaceutical hydrates is of the utmost importance in the processing and storage of drug products, especially since the crystallization of amorphous drugs during dissolution often reduces their bioavailability. Temperature and humidity effects have been studied for decades & many pharmaceutical hydrates and solvates show dehydration or rehydration behavior that affect their properties & stability. However, what is not understood are the kinetics involved in the hydration process and the degree of atomic and molecular disorder created during these solid-gas reactions. Here we present in-situ high energy x-ray measurements of humidity induced effects in two amorphous pharmaceutical hydrates, namely indomethacin and carbamazepine, where dissolution rates of different phases vary. Experiments performed on a custom-built humidity chamber are described together with the effects on amorphous structure over time as the materials swell and ultimately crystallize.
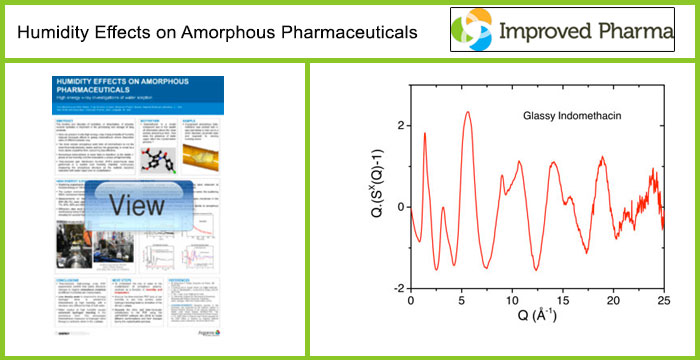
Indomethacin is a model compound due to the wealth of information about the formation and characterization of the more soluble amorphous form and the effect of humid storage conditions. However, the more soluble amorphous solid form of indomethacin is not the most thermodynamically stable and the propensity to revert back to the stable crystalline form under high temperature and humidity results in the drug becoming less effective. Here we describe in-situ bulk measurements on amorphous pharmaceuticals exposed to varying humidity levels using high energy x-ray diffraction. Experiments performed as a function of relative humidity show how surface water is absorbed into amorphous Indomethacin prior to crystallization at the molecular level.