In February 2021, the FDA issued a guidance aimed at preventing unacceptable levels of nitrosamine impurities in pharmaceutical products. This guidance required a risk assessment to be completed by March 31, 2021. This is because nitrosamines are genotoxic and are classified as possible or probable carcinogens.
The guidance also described conditions under which nitrosamines can contaminate drug products. Nitrosamines have been found in ARBs (like valsartan), ranitidine, nizatidine, and metformin. The presence of N-nitrosodimethylamine (NDMA) in ranitidine and nizatidine is due to chemical reactions of these drugs. In contrast, the presence of NDMA in ARBs and metformin is due to contamination from solvents or other sources.
Further, for ranitidine, FDA accelerated stability studies demonstrated that NDMA increased with time, presumably due to solid-state reactions. This establishes that some drugs are intrinsically prone to nitrosamine formation.
The exact reaction for forming nitrosamines is not apparent. The FDA guidance shows the following reaction:
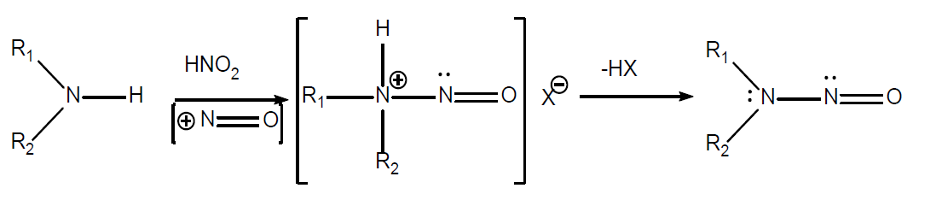
However, a recent review in the Journal of Organic Chemistry shows a slightly different reaction occurring in chlorinated water containing nitrite salts:
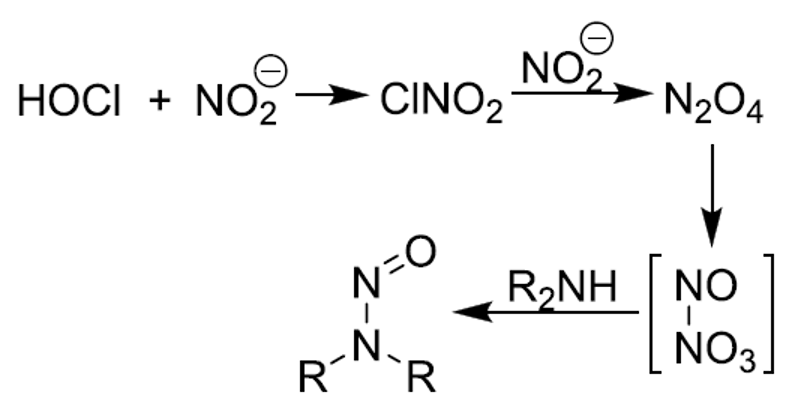
The FDA guidance states that the formation of nitrosamines is possible in the presence of secondary, tertiary, or quaternary amines and nitrite salts under acid conditions. Nitrite salts are widely used in synthetic chemistry and are widespread in the environment. Amide solvents are another source of secondary amines that can react to form nitrosamines. For example, dimethylamine may be an impurity in dimethylformamide. Trimethylamine may also contain secondary amines that can form nitrosamines. The guidance states the amide impurity level needs to be determined for each API. The FDA has also observed: (1) Nitrosamine contamination in fresh solvents; (2) The presence of sodium nitrite impurities in starting materials; (3)Secondary amines as impurities in some raw materials; and (4) cross-contamination with nitrosamines. The guidance also describes several other reaction steps that can cause nitrosamine formation.
The FDA also suggests that nitrite impurities in excipients can react with secondary and tertiary amines and form nitrosamines. This type of reaction could occur in drug products via a solid-state process.
The FDA suggests that the supply chain for all raw materials is an essential factor in preventing nitrosamine contamination. They also state that recovered solvents may pose a risk for nitrosamine impurities.
In the end, the FDA recommended that manufacturers:
- Assess the risk of nitrosamine impurities in APIs, marketed products, and products under approved and pending applications. Risk assessments should be conducted in a timely manner based on the prioritization of drugs.
- Conduct confirmatory testing when there is any risk for the presence of nitrosamine impurities. Due to nitrosamines’ physicochemical properties (low molecular weights, some volatility, and high toxicity), the analytical methods for nitrosamines need specificity, excellent chromatographic separation, and highly sensitive detection capability.
- If nitrosamines above the LOQ are detected, the manufacturer (API or Drug Product) should develop an appropriate control strategy to ensure the nitrosamine remains below the level of 26.5 ng/day. The manufacturer should report changes implemented to prevent or reduce nitrosamine impurities in APIs and drug products to FDA. This includes submission of any drug master file (DMF) amendments in accordance with 21 CFR 314.420(c) and changes to approved applications as required under 21 CFR 314.70 and 314.97 and pending applications under 21 CFR 314.60 and 314.96.
Improved Pharma LLC has the organic chemistry, analytical chemistry, and solid-state chemical expertise to help companies accomplish the entire range of FDA recommendations, including risk analysis and mitigation of risks related to nitrosamines. Improved Pharma is especially skilled in developing extraction methods, crystallization methods, and salt chemistry methods that can remove nitrosamines. Contact us for an assessment of your situation and a science-based approach to solving problems related to nitrosamines.
