Dr. Chris Benmore of Argonne National Laboratory delivered an insightful talk at the 4th Spring Pharmaceutical Synchrotron X-Ray Powder Diffraction Workshop held June 10-11, 2024, at the Novartis campus in Basel, Switzerland. His presentation entitled “Emprical Potential Structure Refinement” focused on strategies for the structural analysis of co-processed compositions, drawing from a pair distribution function (PDF) study of glassy carbamazepine.
Background
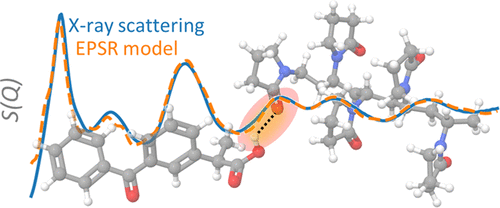 The method described and utilized in the carbamazepine paper is Empirical Potential Structural Refinement (EPSR) of the Pair Distribution Function derived from the Synchrotron measurement of an amorphous composition. The refinement is based on Lennard-Jones potentials and proceeds as follows: 100 or more molecules of drug or drug and polymer are placed in a cubic box with a fixed density.(Benmore et al., 2022) A simulated polymer with only a few repeats of the polymer unit are used to simulate the polymer, if required. The ratio of the drug to the polymer can be controlled. A Monte Carlo method using the Lennard-Jones reference potentials calculates theoretical arrangements of the amorphous molecules. The various Monte Carlo packing arrangements are converted to PDFs and compared to the experimental PDF until the best fit is obtained. In some cases, computational methods are used to improve the fit.
The method described and utilized in the carbamazepine paper is Empirical Potential Structural Refinement (EPSR) of the Pair Distribution Function derived from the Synchrotron measurement of an amorphous composition. The refinement is based on Lennard-Jones potentials and proceeds as follows: 100 or more molecules of drug or drug and polymer are placed in a cubic box with a fixed density.(Benmore et al., 2022) A simulated polymer with only a few repeats of the polymer unit are used to simulate the polymer, if required. The ratio of the drug to the polymer can be controlled. A Monte Carlo method using the Lennard-Jones reference potentials calculates theoretical arrangements of the amorphous molecules. The various Monte Carlo packing arrangements are converted to PDFs and compared to the experimental PDF until the best fit is obtained. In some cases, computational methods are used to improve the fit.
Previous EPSR Study on Amorphous Drugs
We previously reported the results of an EPSR study on liquid and glassy carbamazepine, focusing on the calculated number of tetramers and pentamers in the amorphous phase.(Benmore et al., 2022) The EPSR study showed a higher number of multiple associated molecules in the amorphous phase compared to the liquid phase, which had higher numbers of monomers. Additionally, hydrogen bonding increased by 50% in the glassy phase compared to the liquid, confirmed by SSNMR. The EPSR also revealed pi-pi interactions and dimers and trimers held together by hydrogen bonds.
Benmore’s Strategy for Co-Processed Amorphous Dispersions
Dr. Benmore and coworkers summarized an extension of this EPSR strategy to determine the structure of co-processed drug-polymer systems, with a focus on amorphous solid dispersions.(Wilke et al., 2024) In this study, an amorphous solid dispersion of ketoprofen and polyvinylpyrrolidone (PVP) was prepared by five different methods: melt quenching, rotary evaporation, spray drying, acoustic levitation of a drug-polymer solution, and as controls, a physical mixture. The PDF of each composition was analyzed using the EPSR method and compared.
Findings
- The largest differences among the PDFs were for the rotary evaporated sample, possibly due to solvent retention.
- Differences in local order were observed also potentially related to solvent content.
- The PDF of the amorphous dispersion prepared by spray-drying ketoprofen and PVP solutions into an acoustic levitator was similar to the physically mixed samples.
- It is noteworthy that an earlier study by Improved Pharma compared PDFs of a drug-polymer dispersion prepared by spray drying from different solvents under slightly different conditions, finding that these co-processed materials were very similar.
Further Investigation
In another study, Wilke, Benmore, and coauthors, including Stephen Byrn, investigated amorphous ketoprofen-PVP dispersions with drug loadings ranging from 0 to 75%, prepared by melt quenching at 120 °C (Wilke at al., 2024). Zhuo recently reported a computational analysis of ASDs and found that both reduced steric hinderance and hydrogen bonding were responsible for reduced stability.(Zhuo et al., 2024). We found that the extent of ketoprofen-PVP hydrogen bonding did not significantly change with drug loading, suggesting that hydrogen bonding was not responsible for decreased ASD stability towards crystallization as drug loading increased. Instead, higher drug loading led to decreased steric hindrance of ketoprofen molecules, which may correlate with reduced stability. Additional studies are needed to further test this hypothesis.
Conclusion
The use of empirical potential structure refinement to model the pair distribution function and provide structural information represents a new strategy for analyzing co-processed materials. Combining techniques like those discussed could yield even more information on the structure of co-processed systems.
Benmore, C. J., Edwards, A., Alderman, O. L., Cherry, B. R., Smith, P., Smith, D., Byrn, S., Weber, R., & Yarger, J. L. (2022). The structure of liquid and glassy carbamazepine. Quantum beam science, 6(4), 31.
Wilke, S. K., Benmore, C. J., Menon, V., Smith, D., Byrn, S. R., & Weber, R. (2024). Molecular structure of ketoprofen-polyvinylpyrrolidone solid dispersions prepared by different amorphization methods. RSC pharmaceutics, 1(1), 121-131.
Wilke. S. K., Al-Rubkhi, A., Benmore, C. J., Byrn, S. R., & Weber, R. (2024) Modeling the structure of ketoprofen-poly(vinylpyrrolidone) amorphous solid dispersions with empircal potential structure refinements of x-ray scattering data. Molecular Pharmaceutics, 2024, https://doi.org/10.1021/acs.molpharmaceut.4c00313
Zhuo, X., Jasiukenaite, I., & Löbmann, K. (2024). β-Lactoglobulin-based amorphous solid dispersions: A graphical review on the state-of-the-art. European Journal of Pharmaceutics and Biopharmaceutics, 114396.
