Achieving control is essential in early-stage pharmaceutical development, not only to meet regulatory expectations but to ensure consistency, high-quality, and reliability of clinical batches. Improved Pharma has developed a comprehensive control strategy that supports both drug substance and drug product manufacturing. This strategy is particularly vital when preparing materials for first-in-human (FIH) clinical trials or addressing early development challenges.
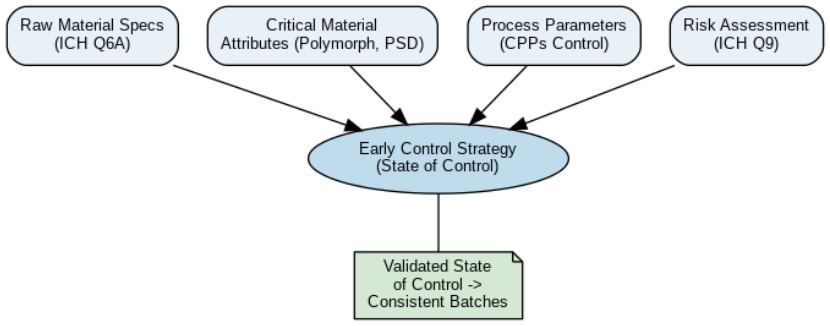
Our approach is grounded in a strong foundation of solid-state chemistry and aligns with international regulatory expectations, including ICH guidelines Q6, Q8, Q9, Q2, Q11, and Q14. This results in a knowledge-driven, risk-managed methodology that identifies and controls critical material attributes (CMAs), critical quality attrtibutes (CQAs), and critical process parameters (CPPs) through the design and development of drug substance and drug product composition, process and test methods.
From Expertise to Action: Building the Control Strategy
A comprehensive control strategy, built on a knowledge-based rationale, is central to our development work. This involves identifying the key variables that influence product performance and establishing early specifications and established conditions (ECs) that deliver consistent control.
We begin by examining CMAs and CQAs, such as polymorphic form and particle size, which can impact dissolution, stability, and bioavailability. Our solid-state expertise enables us to screen polymorphs, identify the most stable and bioavailable form, and implement appropriate control strategies using standard analytical techniques like XRPD and particle size analysis. These controls are aligned with ICH Q6A decision trees and specifications to ensure each batch meets defined criteria.
In parallel, we identify CPPs across unit operations – such as blending time, milling screen size, granulation moisture, and tablet compression force – to ensure reproducibility and quality. For both drug substance and drug product, we design small-scale design of experiments (DoE) to define acceptable operating ranges. These experiments support early understanding of process robustness and ensure that manufacturing is ready to scale. When applicable, we also identify critical formulation parameters essential for nanoparticle processes and processes involving small peptides and nucleic acids – such as lipid or polymer composition, nanoparticle size distribution, encapsulation efficiency, payload concentration, mixing rates, and particle stability – to consistently achieve target product profiles (TPPs), solubility, and stability. Small-scale DoE studies are systematically designed to define acceptable operating ranges, providing early insights into formulation robustness. These studies facilitate effective scale-up, ensuring reliable manufacturing of the therapeutics for entry into clinical studies.
Integrating Raw Material Control and Stability
A successful control strategy must also account for raw material quality. We develop working specifications for active pharmaceutical ingredients (APIs) and excipients that cover identity, potency, polymorph form, moisture content, and other critical attributes. We also assess the variability of materials from different suppliers to understand their impact on product performance and ensure supply chain flexibility without compromising quality.
Early stability studies – both physical and chemical – are essential to confirm that the selected solid-state form maintains its integrity throughout shelf-life. Our laboratory is equipped with tools such as LC-MS/MS for degradation analysis, ensuring rapid identification and quantification of any instabilities.
Managing Risk and Maintaining a State of Control
Improved Pharma integrates Quality Risk Management (ICH Q9) during IND-enabling development activities. We can facilitate risk assessments – including edge-of-failure testing using extreme parameter values – to stress the product, process and test methods and confirm robustness. These assessments focus on CQAs, such as assay, dissolution, and degradation products, helping to define the freedom-to-operate ranges that ensure consistent quality even as development scales up.
The goal is to achieve and maintain a state of control—a condition where the manufacturing process consistently produces material that meets specifications. This state supports not only compliance, but also operational efficiency and development speed.
Extending to Complex Products and Protecting IP
Our science-driven approach extends beyond conventional oral dosage forms to encompass complex modalities, including nanoparticles and amorphous dispersions, where control of attributes like particle size distribution and surface charge is critical.
Moreover, by identifying and controlling solid-state properties early, we help clients secure valuable intellectual property (IP) – for example, by patenting stable polymorphic forms or novel formulation designs. This early work strengthens the product’s overall regulatory and commercial position.
Conclusion: A Strategy That Supports Speed, Quality, and Flexibility
By implementing a robust, science-based control strategy in early development, Improved Pharma helps clients:
- Achieve sameness across clinical batches even when manufactured at different scales
- Lay a foundation for Quality by Design (QbD) and efficient scale-up
- De-risk IND clinical supply production by maintaining a state of control
- Protect the product with IP strategies based on deep materials knowledge
- Enable freedom to operate across suppliers and manufacturing configurations
Our holistic strategy combines practical implementation with deep technical insight. Whether you’re preparing for first-in-human trials or solving an early-stage development challenge, Improved Pharma can help you move forward with confidence.
Diagram: Early Development Control Strategy Framework
Below is a simplified framework that illustrates the components of a control strategy in early development: