Single-crystal X-ray diffraction (SCXRD) provides the most definitive structural picture of pharmaceutical solids. From a single crystal, we obtain the unit cell, space group, and full atomic coordinates, which reveal hydrogen-bonding networks, π–π (ring) stacking, protonation states, crystal packing, solvation, stoichiometry, absolute configuration, and the presence or absence of disorder. With these data, we can unambiguously define polymorphs and hydrates/solvates, understand how form relates to properties such as stability, tabletability, and crystal fracture, and establish a solid foundation for regulatory and intellectual property strategies.
Modern SCXRD is fast. With current detectors and software, structures are often solved and refined in under a day, with formal reports typically following within one to two days. This speed is especially valuable during form screening: crystals can be plucked directly from a crystallization experiment or a microscope slide, analyzed immediately, and the resulting structures used to steer the next round of screening. The combination of SCXRD with optical microscopy enables rapid mapping of a compound’s polymorph landscape.
SCXRD also links cleanly to powder diffraction. From an accurate single-crystal model, we can calculate a reference PXRD pattern, which is then used to identify forms in bulk samples, flag mixtures, and detect trace levels of alternate forms. Powder patterns comprised of mixtures can be quantitated through Rietveld refinement when the SCXRD structures of all components are known. In problematic cases, the single-crystal model helps interpret powder patterns affected by preferred orientation or disorder, clarifying whether a new pattern reflects a genuinely new form or a disordered variant of a known one. For salts and hydrates, SCXRD provides precise ion-pair geometries and water/solvent positions, which in turn explain differences in stability and performance.
Because SCXRD unveils molecular packing motifs, it also guides salt and co-crystal design. Observed synthons and packing interactions suggest promising counterions or coformers, inform targeted screens, and support crystal-structure prediction efforts by supplying validated packing models.
For decades, SCXRD has illuminated the chemical and physical stability of solids by showing which functional groups are exposed on the crystal surface, how tightly molecules pack, and which interactions dominate cohesion. That information explains the differences in reactivity with moisture or gases, tendencies toward phase transformation, and mechanical behaviors that are relevant during manufacture.
In many cases, SCXRD can determine absolute configuration, providing direct evidence for chirality when anomalous dispersion is sufficient—an important point for chiral APIs and their salts.
Examples Of SCXRD In The Analysis Of Polymorphs And Hydrates
SCXRD is perhaps the most powerful method for identifying and understanding polymorphs. One of the classic case studies is the family of “color polymorphs” in the compound 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile, known as ROY for its red, orange, and yellow forms. Early work showed at least three distinct forms—red, orange, and yellow—and single-crystal diffraction provided their definitive structures.[1] Those structures established that the different colors arise from changes in molecular conformation, especially the dihedral angle between the phenyl ring and the thiophene ring, which in turn alters conjugation and electronic transitions. Since those first reports, more than a dozen additional ROY polymorphs have been discovered and identified by SCXRD. Derivatives of ROY have also been synthesized and exhibit color polymorphism. Across these studies, SCXRD, supported by vibrational spectroscopy and other techniques, supplied the decisive evidence that the color variants are true polymorphs with distinct crystal structures.

Figure 1. The red, orange, and yellow polymorphs of ROY first crystallized and analyzed by SCXRD at Purdue University circa 1994[1]
SCXRD is also invaluable for distinguishing genuine polymorphism from variability caused by disorder. A well-known example is aspirin: single-crystal studies combined with diffuse-scattering analysis have shown that the so-called “Form II” can be interpreted as stacking-faulted domains within the Form I lattice, rather than a fully independent polymorph.[2] This kind of crystallographic insight prevents misclassification and keeps solid-form control grounded in structure, not just powder patterns.
Hydrates provide another arena where SCXRD studies shine. Classic analyses categorize hydrates into channel (or tunnel) hydrates, isolated-site (lattice) hydrates, and hydrates associated with metal ions.[3] The structural diversity is striking. Some systems—caffeine is a textbook example—contain continuous water channels threading the lattice, and channel hydrates typically show partial occupancy of the channels, with humidity-dependent water content.[4] Among β-lactam antibiotics, channel hydrates are common and instructive: cefadroxil monohydrate forms a channel-type structure that can dehydrate stepwise without catastrophic lattice collapse, with water occupancy in the channels varying with humidity;[5] cefazolin sodium pentahydrate contains water in two environments, including large solvent tunnels and coordination regions near sodium and the tetrazole moiety, and it readily loses the more labile “tunnel” water; and cephalexin is a well-documented channel hydrate with an average water content near 1.9 H₂O per molecule, reflecting variable channel occupancy. In each case, single-crystal structures pinpoint where water resides, how it interacts with the host lattice, and why the materials absorb or desorb water as conditions change. Without single-crystal diffraction, this level of understanding would be far harder to achieve.
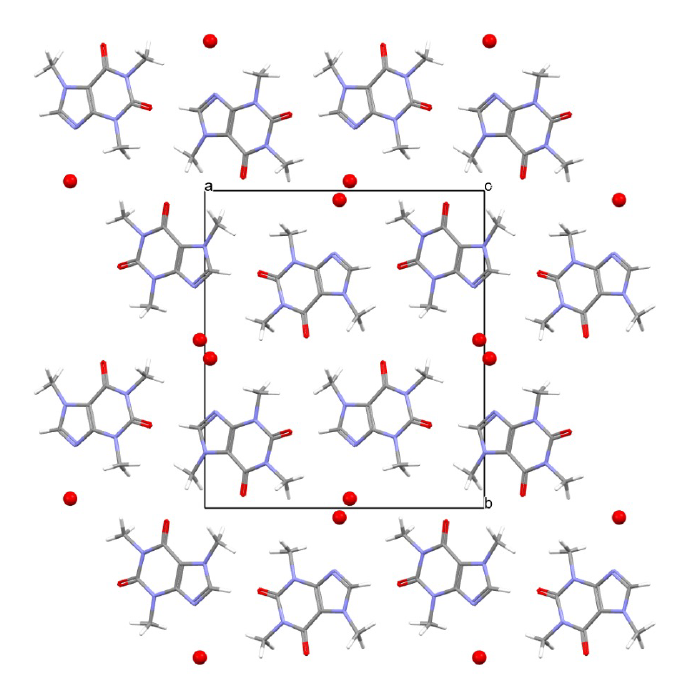
Figure 2. SCXRD structure of caffeine hydrate viewed down the z-axis showing tunnels of water molecules (red) running through the lattice in the z direction.[4]
Another critical contribution of SCXRD is defining the location of hydrogen atoms in systems where it is unclear whether a solid is a salt or a co-crystal. Establishing proton positions—which donates and which accepts—settles the question definitively and prevents regulatory or IP ambiguity. A particularly clear discussion of this approach was published in 2007 by SSCI.[6] In challenging, dynamic, or disordered systems, combining single-crystal X-ray data with solid-state NMR provides a more comprehensive understanding of local mobility, disorder, and proton environments, offering a more complete picture than either method alone.
Concluding Remarks
Once an SCXRD structure is known, the calculated powder diffraction pattern becomes a powerful practical tool. It helps identify mixtures, diagnose preferred orientation, select the most distinctive lines for analytical methods, and support intellectual-property claims with unambiguous structural references. PXRD peaks are often defined in intellectual property claims and accurate relative intensities should be confirmed, most commonly through comparison to the calculated PXRD pattern, before designating any PXRD peaks as representative of a given form. In sum, single-crystal X-ray diffraction remains the gold standard for defining polymorphs, hydrates, and other solid forms, and it is critically important for understanding stability, guiding development and control strategies, and strengthening both regulatory submissions and intellectual property.
From a regulatory perspective, SCXRD structures are the gold standard for defining solid forms in submissions. They anchor form designations, support specifications, and reduce ambiguity in comparability and lifecycle filings. They also underpin IP, supplying unambiguous structural definitions for polymorph claims and enabling calculated powder patterns for claim support.
At Improved Pharma, we pair rapid SCXRD with form screening, PXRD confirmation, and stability assessment to deliver decision-ready data. We also transfer intellectual-property rights in the results to our clients, so SCXRD reports, coordinates, and calculated patterns can be used directly in regulatory dossiers and patent filings.
Bottom line: SCXRD turns microscopic crystals into macroscopic certainty—clarifying form, guiding development, and strengthening both quality and IP.
Recently, Dr. Jared Smit joined our team of experts at Improved Pharma to lead our R&D effort in SCXRD. For more information on Improved Pharma’s SCXRD services, please contact Jared at jared.smit@improvedpharma.com.
References
- Stephenson, G.A., Borchardt, T.B., Byrn, S. R., Bowyer, J., Bunnell, C.A., Snorek, S.V., Yu, L., Conformational and color polymorphism of 5-methyl-2-[(2-nitrophenyl) amino]-3-thiophenecarbonitrile. J. Pharm. Sci. , 1995. 84: p. 1385– 1386.
- Bond, A.D., Boese, R., Desiraju, G. R., On the Polymorphism of Aspirin: Crystalline Aspirin as Intergrowths of Two “Polymorphic” Domains. Angew. Chem. Intl. Ed., 2007. 46: p. 618-622.
- Morris, K.R., Rodriguez-Hornedo, N., Classification of Hydrates, in Encyclopedia of Pharmaceutical Technology, J. Swarbrick, Boylan, J.C., Editor. 1993, Marcel Dekker. p. 393-440.
- Byrn, S.R., Lin, C.T., The Effect of Crystal Packing and Defects on the Desolvation of Hydrate Crystals of Caffeine and L(-)-1,4-cyclohexadiene-1-alanine. J. Am. Chem. Soc., 1976. 98: p. 4004.
- Brittain, H.G., Fluorescence studies of the dehydration of cefadroxil monohydrate. Journal of pharmaceutical sciences, 2007. 96(10): p. 2757-2764.
- Childs, S.L., G.P. Stahly, and A. Park, The salt− co-crystal continuum: the influence of crystal structure on ionization state. Molecular pharmaceutics, 2007. 4(3): p. 323-338.
