Steve Byrn, September 26, 2025
Reduced dose formulations provide one of the most exciting approaches to improving medicines by reducing side effects, pill burden, and drug load in the patient and by reducing cost of manufacture. Improved Pharma has extensive experience in developing reduced-dose pharmaceutical products and offers a range of services to support such development.
Historically, reduced-dose products have been of interest since the 1950s. One of the earliest and most influential contributors was the well-known pharmaceutical scientist Eino Nelson, who pioneered the use of salt formation to enhance the bioavailability of drugs such as theophylline for asthma. His work demonstrated that salt formation could increase a drug’s solubility by more than 1,000-fold in favorable cases. This improved solubility resulted in enhanced bioavailability and enabled the development of lower-dose formulations.
In the 1960s and 1970s, additional landmark studies further advanced this field. For example, griseofulvin was reformulated using micronization to increase surface area and dissolution rate, effectively reducing the required daily dose from nearly 1 gram to less than 500 mg. Likewise, digoxin was reformulated into soft gelatin capsules, allowing more consistent absorption and downward dose adjustments. In the case of phenytoin, used to treat epilepsy, improvements in particle size control and salt selection led to better absorption and more predictable therapeutic effects.
FENOFIBRATE: A CASE STUDY IN REDUCED-DOSE FORMULATIONS
Fenofibrate is perhaps the most illustrative example—or “poster child”—of reduced-dose formulation development. It is a fibric acid derivative used to lower cholesterol and triglyceride levels. Fenofibrate is highly lipophilic, practically insoluble in water, and exhibits low oral bioavailability. The original formulation required a high dose of 300 mg per day and had to be taken with meals to ensure absorption.
Over time, through a series of formulation innovations, the required dose was reduced by approximately half, and food effects were minimized or eliminated. These changes were achieved using advanced pharmaceutical technologies, as summarized in Table 1.
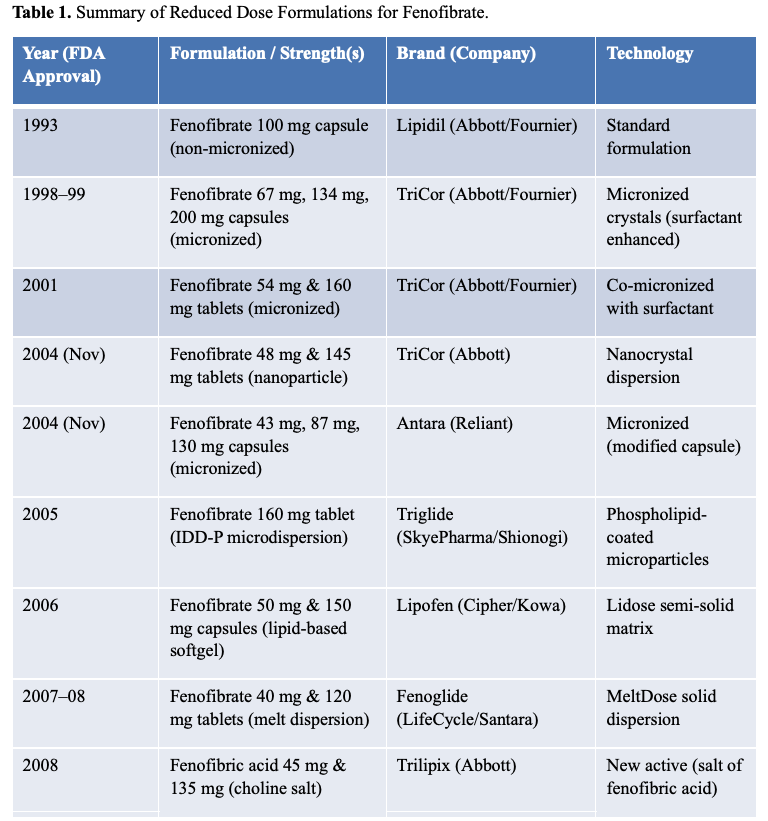
Formulation Evolution of Fenofibrate
Table 1 outlines the historical development of fenofibrate formulations, showing the year of FDA approval, strength, brand, and technology:
- 1993: Abbott and Fournier introduced a 100 mg capsule; the typical dose was three capsules daily (total 300 mg).
- 1998/1999: A micronized formulation with surfactant was introduced (Tricor® 67 mg), reducing the dose while maintaining bioavailability.
- 2001: Abbott/Fournier released a 54 mg formulation, which supported a once-daily 160 mg tablet.
- 2004: Abbott introduced a Nanocrystal dispersion (Tricor® 48 mg), further enhancing solubility.
- 2004: Reliant Pharmaceuticals launched Antara®, a branded generic with micronized fenofibrate (43 mg).
- 2005: SkyePharma and Shionogi introduced Triglide®, a phospholipid-coated nanoparticle (50 mg).
- 2006: Cipher and Kowa developed Lipofen®, using Lidose® technology (semi-solid lipid matrix) in 50 mg and 150 mg capsules.
- 2008: LifeCycle Pharma introduced Fenoglide® (40 mg and 120 mg) using melt dispersion technology.
- 2012: Abbott launched Trilipix®, a fenofibric acid choline salt, dosed at 45 mg, marking a shift to a new salt form.
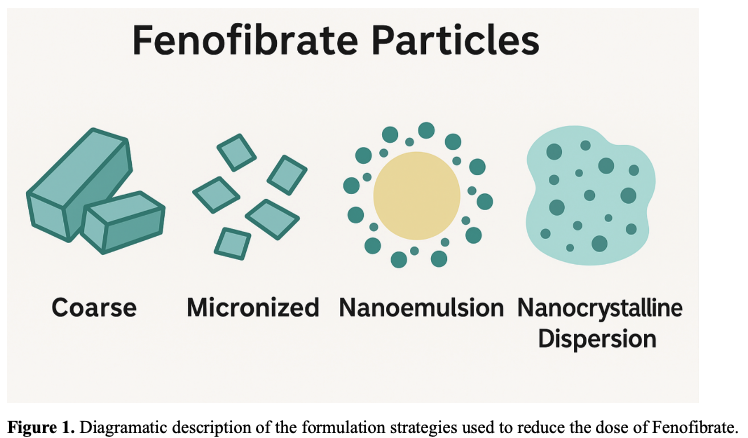
These successive innovations demonstrate multiple strategies—micronization, lipid-based formulations, nanocrystals, dispersions, and salt switches—to reduce dose and improve bioavailability. Figure 1 visually illustrates the structural differences between several of these formulations.
Patent Landscape of Fenofibrate Formulations
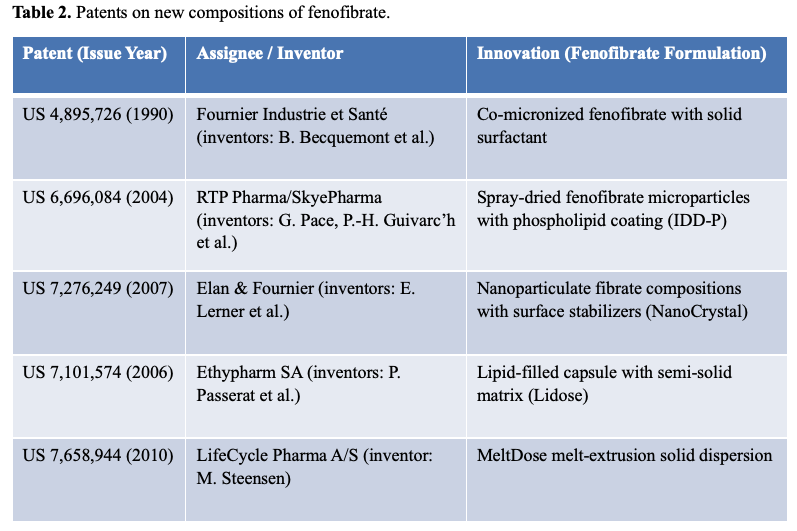
Clearly, these new compositions would result in intellectual property, and several of these innovations were protected by US patents, as summarized in Table 2:
- US 4,895,726 (Fournier, 1990): Covers co-micronized fenofibrate with surfactants; broad claims that may extend to later formulations.
- US 6,696,084 (SkyePharma): Covers spray-dried fenofibrate microparticles with phospholipid coating, such as Triglide®.
- US 7,276,249 (Fournier, 2007): Covers nanoparticulate fenofibrate stabilized with surface modifiers (Nanocrystal technology).
- 2006 Patent: Covers Lidose® technology—lipid-filled capsules developed by Cipher/Kowa.
- US 7,658,944 (LifeCycle Pharma, 2010): Covers melt dispersion technology, used in Fenoglide®.
It is important to note that the filing and grant dates of patents often lag behind product approval dates, due to the time it takes for review and prosecution at the patent office. Additionally, while these patents appear to correspond with products on the market, patent coverage of commercial products is ultimately subject to legal interpretation and court decisions.
It is interesting to review the currently marketed fenofibrate drug products listed on Drugs@FDA. The original micronized fenofibrate formulation continues to be available on the market, with 11 products still labeled as “micronized fenofibrate.” In total, over 25 generic fenofibrate products are currently on the market, along with more than 10 fenofibric acid products.
Some branded products have been discontinued:
- Fenoglide and Lepido have both been discontinued.
- Lipofen remains on the market.
- Tricor, one of the original formulations, has been discontinued, as have Tricor Micronized and Triglide.
In contrast, Trilipix (the choline salt form of fenofibric acid) is still on the market, and more than 10 generics of Trilipix are also available.
This large number of branded and generic products, more than 50, is typical for important drugs and reflects the current landscape of pharmaceutical marketing and lifecycle management.
Conclusion: Fenofibrate represents a textbook case of dose reduction through formulation. Over two decades, multiple companies introduced new technologies to make new compositions that improved solubility and enabled lower daily doses. This history illustrates the power of formulation science, not only to improve patient adherence and therapeutic consistency, but also to support market differentiation and intellectual property protection. Improved Pharma remains abreast of these types of improvements and can provide assistance in preparing new compositions with reduced dose and improved patient experiences.
