
Thermodynamic stability and form selection.
Form selection of active pharmaceutical ingredients is regarded as an essential part of drug development. The goal of form selection is to identify the most suitable solid form for toxicological studies, drug product development, and eventually commercialization. Determining the order in which solid forms exhibit physical stability is one of the key elements for form selection, since the form selected should maintain acceptable physical stability during product manufacturing and storage. Chapter 21 of Solid-State Properties of Pharmaceutical Materials by Byrn, Zografi, and Chen (Wiley, 2017) presents various screening methodologies, selection strategies, and processes by which final decisions are generally made.
Considerations in Determining the Transition Temperature of Enantiotropic Polymorphs with Slow Transformation Kinetics were previously discussed. We now focus our attention on slurry experiments and the approaches that can be used to increase their effectiveness to confirm the relative stability of polymorphs or anhydrate/hydrate.
The solution-mediated polymorph transformation.
The difference in free energy between solid phases is directly proportional to their relative solubilities. Therefore, the order of solubility and dissolution of a solid form at a specific temperature will directly reflect the order of thermodynamic stability at that temperature.
Haleblian and McCrone, in their classic 1969 review1, described using solution-mediated phase transformations to determine the most stable form. They referred to a 1965 paper on the explosive HMX using a specially constructed apparatus. They utilized a microscope and a drop of solvent and observed which form grew and which form disappeared. The method was not widely used in the 1980s. Then in the early 1990s, SSCI, Inc. reintroduced the method and, instead, analyzed the conversion by X-ray powder diffraction.
The mechanism of a slurry experiment.
Slurrying is a popular technique to screen for stable polymorphs and hydrates. Slurry experiments are carried out by mixing the sample with enough solvent to make a suspension. The suspension is constantly agitated for days or even a longer time, until the solid is harvested from the suspension for physical characterization.
The solubility difference between forms drives the solution-mediated transformation that generally occurs in a slurry experiment. The transformation can be divided into three steps:
- dissolution of the metastable phase to form a solution which is super-saturated with respect to the more stable phase
- since super-saturated solutions are metastable, a more stable phase will eventually crystallize to establish equilibrium and remove supersaturation2-3
- the more stable phase will grow at the expense of the metastable form.
Thermodynamics dictates that the most stable phase will eventually crystallize, with crystallization being slower than dissolution, and the nucleation rate usually slower than the rate of crystal growth during crystallization. In practice, the path and the timescale for the transformation are governed by the kinetics of the conversion and any step could be rate limiting.
Why wait for nucleation?
The free energy barrier for nucleation may be difficult to overcome. It is necessary to find conditions that provide a sufficient driving force.
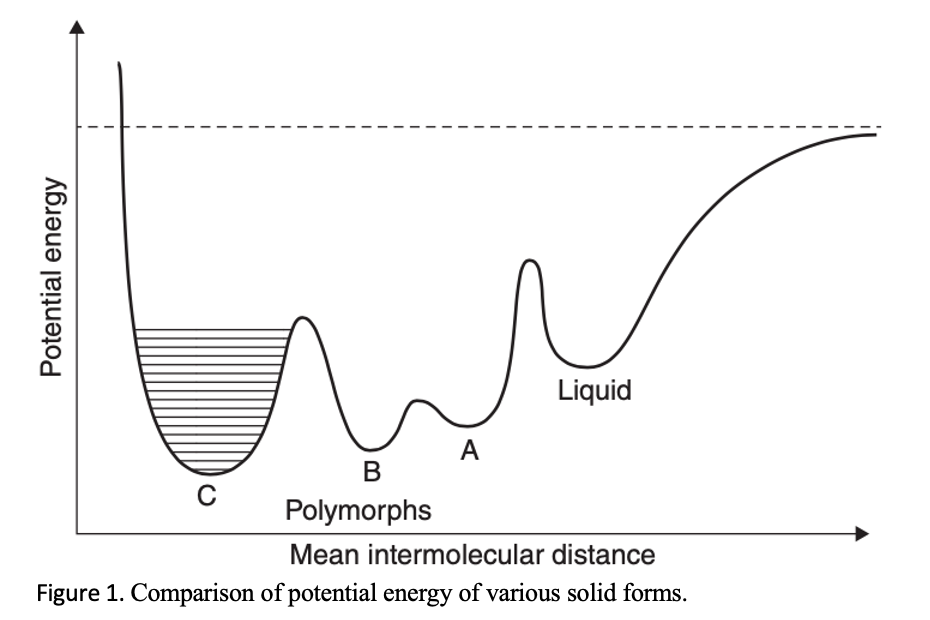
The degree of agitation and temperature can help change the polymorphic transformation rate by influencing the crystallization kinetics of the more stable polymorph. Moreover, if the different polymorphs are already available through other crystallization methods, then seeding is a luxury that should utilized. More advantageous is to use an equal mixture of two polymorphs or an anhydrate/hydrate pair. Even a slight increase in the amount of one component and the decrease of the other component will indicate which form is more stable under the slurring condition, temperature, and solvent.
Achieve higher solubility.
The solubility of the compound in the solvent system and the nature of the solvent-solute interactions may limit the transformation rate. Commonly, without higher solubility and weak solvent-solute interactions to promote the transformation, it will not occur over the time course of the experiment. Solvents or solvent mixtures that give solubilities sufficient to achieve solute concentrations greater than 8 mM appear to favor conversion to a more stable form.4-5
Improve the impurity profile of the API.
The rate of conversion is also dependent on the impurity profile. Impurities, especially those that are structurally related, may stabilize the metastable forms by retarding the solution-mediated transformation.6 Even in trace amounts, impurities may act as a nucleation or growth inhibitors.7 Such impurities may slow the overall conversion to the point that a stable form is missed in early screening. The use of a different solvent may remove the inhibitory effect of an impurity due to an increase in the solubility of the compound. Another mitigation strategy is to use less solid in the slurry so that the level of impurity in the solution can be reduced.8 In general, the use of a large number of solvents with varying chemical properties (e.g. dipole, hydrogen bonding, water activity, boiling point) will have a better chance of finding a more stable form. API lots with different levels of purity should also be studied to see the effects of impurities.
Consider interactions with excipients in drug product formulations.
Through similar mechanisms described for impurities above, excipients and/or additives may also selectively nucleate the metastable form and even possibly stabilize the form within the formulation or suspension. The influence of additives, excipients, or solvents on polymorph selection is well established in the literature. It is suggested, further, that intended excipients and/or process-related solvents are utilized in slurry experiments to determine if they promote the formation of metastable forms of the API.
Do not let slurry experiments lead you astray.
Switching solids forms during development is not desirable, but sometimes it is inevitable due to the emergence of a new stable polymorph, or the need of a different form to enhance stability, solubility, processibility, or intellectual property. Improved Pharma can help with form selection at various stages of development, as early as drug candidate selection and later as part of life cycle management after commercialization.
Want to find out more?
Contact us at info@improvedpharma.com or explore how we improve pharmaceutical methods, formulations, and processes.
Author: Stephan Parent, Solid-State Pharmaceutical Research Advisor at Improved Pharma. This article has been adapted from Solid-State Properties of Pharmaceutical Materials by Byrn, Zografi, and Chen (Wiley, 2017).
- Haleblian, J.; McCrone, W. Pharmaceutical Applications of Polymorphism. Journal of Pharmaceutical Sciences1969, 58 (8), 911–929. https://doi.org/10.1002/jps.2600580802.
- Bernstein, J. Polymorphism in Molecular Crystals; Clarendon Press: Oxford, 2010. https://doi.org/ 1093/acprof:oso/9780199236565.001.0001.
- Brittain, H. G. Polymorphism in Pharmaceutical Solids; Informa Healthcare: New York, 2009.
- Gu, C.-H.; Young, V.; Grant, D. J. W. Polymorph Screening: Influence of Solvents on the Rate of Solvent-Mediated Polymorphic Transformation.Journal of Pharmaceutical Sciences 2001, 90 (11), 1878–1890. https://doi.org/10.1002/jps.1137.
- Miller, J. M.; Collman, B. M.; Greene, L. R.; Grant, D. J. W.; Blackburn, A. C. Identifying the Stable Polymorph Early in the Drug Discovery–Development Process.Pharmaceutical Development and Technology 2005, 10 (2), 291–297. https://doi.org/10.1081/pdt-54467.
- Lancaster, R. W.; Karamertzanis, P. G.; Hulme, A. T.; Tocher, D. A.; Lewis, T. C.; Price, S. L. The Polymorphism of Progesterone: Stabilization of a “Disappearing” Polymorph by Co‐Crystallization.Journal of Pharmaceutical Sciences 2007, 96 (12), 3419–3431. https://doi.org/10.1002/jps.20983.
- Gu, C.-H.; Chatterjee, K.; Young, V.; Grant, D. J. W. Stabilization of a Metastable Polymorph of Sulfamerazine by Structurally Related Additives.Journal of Crystal Growth 2002, 235 (1-4), 471–481. https://doi.org/10.1016/s0022-0248(01)01784-5.
- Gong, Y.; Collman, B. M.; Mehrens, S. M.; Lu, E.; Miller, J. M.; Blackburn, A.; Grant, D. J. W. Stable-Form Screening: Overcoming Trace Impurities That Inhibit Solution-Mediated Phase Transformation to the Stable Polymorph of Sulfamerazine.Journal of Pharmaceutical Sciences 2008, 97 (6), 2130–2144. https://doi.org/10.1002/jps.21139.
