A recent paper by Testa et al., Int. J. Pharm., 556 (2019) 125, investigated the mixture analysis of Olanzapine, which crystallizes in more than 60 solid forms, using synchrotron X-ray diffraction. They suggested that synchrotron techniques could help guide the discovery of methods to prepare and analyze pure polymorphs. Their observations and suggestions are consistent with our experience at SSCI and our current problem-solving studies at Improved Pharma.
Improved Pharma has extensively utilized high energy synchrotron radiation via beamline 11-BM-B at Argonne National Laboratory. Our experience shows that synchrotron radiation provides ways to solve complex polymorph mixture problems that are not possible using normal resolution experiments (conventional techniques). In conventional XRPD analysis of complex mixtures, if the peaks are overlapped, it can be very difficult to differentiate between a complex mixture or the appearance of a new form. Furthermore, conventional XRPD often suffers from preferred orientation whereas synchrotron studies avoid that by utilizing spinning capillaries in transmission mode.
In some cases, new forms are present at low concentrations and conventional diffraction shows these forms as mixtures of known forms. Testa showed a pattern similar to patterns we have seen at Improved Pharma utilizing synchrotron analysis.
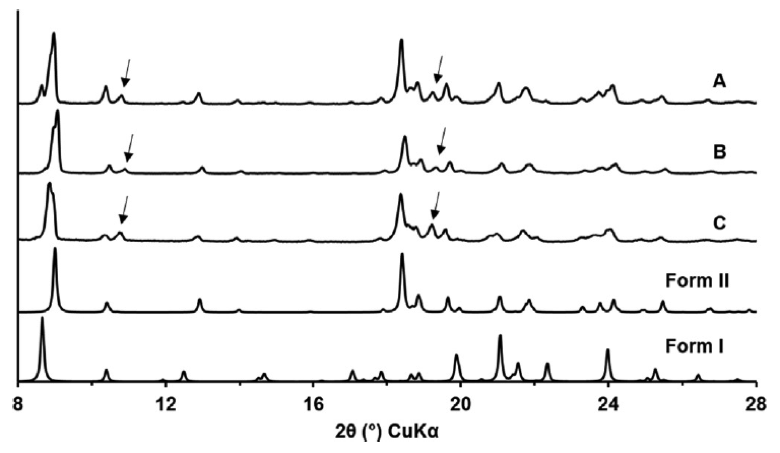
Figure 1. XRPD patterns of OLZ A, B and C and the calculated ones for polymorphous I and II.
The arrows in Testa’s figure represent at least one new Olanzapine form. The appearance of a new form in these samples was substantiated by Solid State NMR studies.
We have seen similar cases at Improved Pharma, where synchrotron analysis enabled the detection of a new form. In one particular case, polymorph screens had been conducted by two different service providers. Both providers used conventional XRPD analysis to characterize the samples. In both studies, only two main polymorphs were found. After production scale-up, a new, weak peak sometimes appeared in certain lots. At first, the new peak was attributed to Form 2, a known form. We analyzed several of these lots with synchrotron XRPD and discovered that the weak peak was not due to Form 2. The excellent resolution of beamline 11-BM-B revealed that a new, third form was present. This new Form 3 had a pattern very similar to that of Form 2 and could be clearly differentiated using synchrotron XRPD analysis. Incorporating synchrotron XRPD analysis into polymorph screen projects would provide a better understanding and increased confidence of the results. As such, we highly recommend supplementing polymorph screens with synchrotron testing.
Synchrotron radiation also provides a way to identify forms present in pharmaceutical formulations. As discussed above, the patterns from a high energy synchrotron measurement provide the resolution and sensitivity to detect small amounts of polymorphs and/or crystalline materials in a formulation For example, Egusa, et al., Drugs R D (2017) 17, 413, utilized synchrotron powder diffraction to detect tiotropium bromide at a significantly lower level, at an order of magnitude lower than conventional powder diffraction This study showed that despite the very large excess of lactose, synchrotron XRPD was clearly able to determine which form of tiotropium bromide was present in the drug formulation. In the conclusion they stated “This method is potentially applicable to other drug substances and dry powder formulations with lower drug concentrations. The achievable level of detection may be lower than reported here, depending on the active pharmaceutical ingredient, its polymorphic forms, particle size distribution, domain size and thus pathway of polymorphic conversion thereof, and pharmaceutical composition.” At Improved Pharma, we routinely utilize synchrotron XRPD to aid in the detection of low amounts of forms in both drug substances and drug products.
In another example, Raj Suryanarayanan at the University of Minnesota used synchrotron radiation to analyze salt disproportionation in a tablet containing pioglitqazone along with microcrystalline cellulose, magnesium stearate, croscarmellose sodium, lactose monohydrate, and sodium chloride. They could clearly see diffraction lines from pioglitazone free base and the formulation components as shown in Figure 2 below.
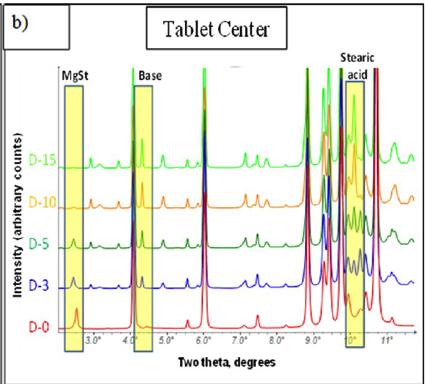
Figure 2. Synchrotron analysis of pioglitazone HCl tablets showing formation of the free base.
For the above reasons, we suggest that in all but the simplest cases, mixture problems should first be addressed using synchrotron radiation. Once a clear understanding of the mixture problem is obtained using synchrotron radiation, it is likely that standard XRPD methods on standard laboratory equipment could be developed for control purposes.
