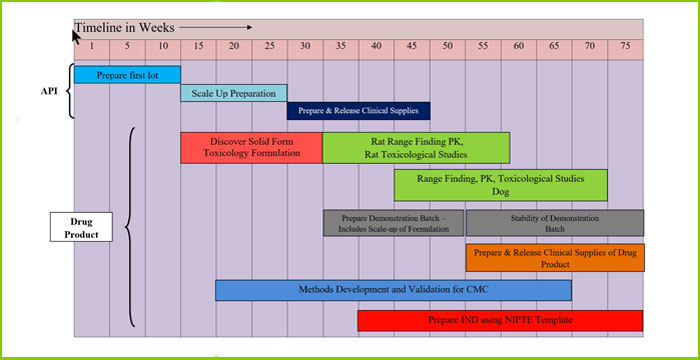
Current approaches to accelerated drug development typically involve parallel track activities utilizing sophisticated project management capabilities. Improved Pharma has developed a strategy that continues to utilize these project management tools but in addition utilizes predictive tools based on structure analysis of formulations and anticipation of major instabilities of formulations by assessing formulation risk. Orphan drugs and especially drugs used for rare diseases such as viral diseases, are typically insoluble. In fact, some estimates suggest that as many as 80% of all drugs under development are poorly soluble. Using an amorphous form is perhaps the fastest approach to combating insolubility, and improving bioavailability and combining an amorphous form with a stabilizing polymer can improve the stability of the formulation. But amorphous forms can crystallize making them difficult and unpredictable to develop. Traditional laboratory approaches are insufficient for understanding the solid state chemistry/physics of the drug:polymer compositions and lead to laborious and time consuming trial-and-error formulation development. Improved Pharma approaches this problem utilizing Synchrotron X-ray technology to analyze the dispersions as well as SSNMR, spectroscopy, and spectroscopic mapping. The Synchrotron technology includes levitated drop (containerless) methods, and Pair Distribution Functions (PDFs) and provides an assessment of drug:polymer structure and their potential stability. The improved insight into the structure and stability of the formulation can lead to improved QbD strategies and stronger intellectual property. Improved Pharma also utilizes both dissolution and animal pharmacokinetic studies, when needed, to accelerate development.
For more information, please see our presentation recently given at the 5th Controlled & Modified Drug Release Summit (Nov 2018)